The first fallacy, that vacuum causes adhesion, was understandable because of Galileo’s influential statement above and also because vacuum was invented by Newton’s contemporary, Galileo’s pupil and secretary Torricelli, who built the original mercury barometer around the time of Newton’s birth, generating the first artificial vacuum in the closed tube above the mercury column. The great force of vacuum on the Earth’s surface was later demonstrated by von Guericke when Newton was 13 years old. It was a tremendous demonstration, where two teams of 8 horses failed to separate the “Magdeburg hemispheres” after the air was withdrawn by von Guericke’s air pump.
We can dismiss the vacuum theory of adhesion easily today, as in Chapters 1 and 2, because astronauts have found that, in space, suction does not give adhesion, whereas the molecular adhesion of particles in space is better than on Earth. We know therefore that the adhesion due to vacuum is only an indirect form of gravitational adhesion, varying from planet to planet. The suction effect cannot explain molecular adhesion.
The second fallacy is more difficult because it arises from the adhesion paradox; that if we break a cup, the parts do not stick together merely by putting the pieces back in contact. Usually, we have to insert some adhesive or glue to join and stick the fragments of pottery back. So we presume that materials do not naturally adhere but require an adhesive layer in between. Similarly, when building a house, we find that the bricks will only stick together if we put mortar in between to make a strong joint, as shown in Fig. 3.2. The same sort of argument is put forward to explain why you cannot make sandcastles from dry sand. You need to use damp sand because the water acts as a glue to hold the sand grains together.
This was the question that Newton puzzled over. If you could get the bricks or the sand grains into molecular contact, he mused, then they should stick. We now know that this is true, because when you take small particles with smooth surfaces, then bring them into close proximity, typically within 10 nm of each other, then the particles leap into contact. All known particles, whether rubber,
|
Figure 3.2. (a) Bricks placed in contact do not adhere unless, (b) a layer of mortar sticks them together. |
gold, mica, or gelatin, do this. It seems that molecular adhesion is a very general and universal phenomenon. Thus the adhesion paradox is really caused by the problem of getting the bodies close enough together in the first place. The molecular adhesion forces are of such short range that they cannot act even over micrometer gaps.
What is more, it has been shown quite clearly that putting water or adhesive on the particles reduces the molecular adhesion.3 Molecular adhesion works best in the vacuum of space. Already on Earth, the atmosphere of oxygen, moisture and organic contamination is known to reduce molecular adhesion by orders of magnitude. The conclusion is inescapable; adhesives reduce the adhesion between clean bodies.
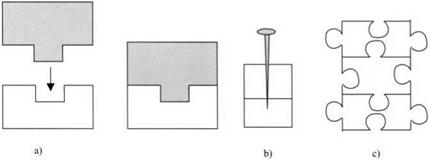
The third fallacy is insidious because it stems again from our common-sense knowledge of making furniture and building cars. It is universally obvious that wooden chairs are best assembled using mortise and tenon joints, as shown in Fig. 3.3(a). A male key is made from one piece of wood and this fits snugly into the female slot in the other piece. If the joint is finely made, then glue is
|
Figure 3.3. (a) Mortise and tenon joints, (b) the nail, (c) jigsaw puzzle. |
unnecessary. The artisan inserts glue anyway for a belt and braces solution. A
cruder example is the nailed wood, illustrating the old adage of adhesive
technology: “When all else fails, use whopping great nails.” Finally, the jigsaw convinces us as infants that mechanical keying is the source of adhesion.
This keying idea has been applied to particles; for example, Evans4 stated, “strength of (powder) compacts results mainly from the interlocking of irregularities on the particle surfaces.” This concept is totally false. The same idea has also been applied to the binding of antigens to antibodies.5 It is a macroscopic idea which we have presumed is also true at the nanometer level. However, it is an idea which cannot be correct. There are no Velcro hooks and eyes at the
molecular level. Newton knew this and dismissed the notion of “hook’d
atoms.” He found that the smoother he polished his glass marbles, the better they adhered. Atomically smooth surfaces stick best of all. These conceptual problems have arisen because we assume that macroscopic behavior applies to molecules. Some critical observations show this to be a false premise.
 5 сентября, 2015
5 сентября, 2015  Pokraskin
Pokraskin 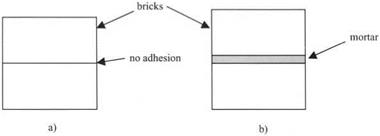
 Опубликовано в рубрике
Опубликовано в рубрике 