Because of the variable and unpredictable nature of adhesion, it has been a common practice through the centuries to adopt a safe approach and design structures with the worst possible scenario in mind. In other words, engineers have traditionally adopted a theory of zero adhesion. This was certainly true of medieval bridge and cathedral builders who desired that only compression forces existed in their constructions, ensuring there were no tensions at the mortar joints to open up cracks. Figure 1.6(a) shows a brick lintel which gives a tension, causing a crack in the mortar adhesive. By devising first the corbelled arch and
|
Figure 1.6. (a) Tension crack in the mortar of a brick lintel, (b) compression stresses preventing cracks in a corbelled arch. |
then the curved arch, these tensions could be overcome through the compressions acting from the ends, as shown in Fig. 1.6(b), relieving any cracking stresses in the mortar.3
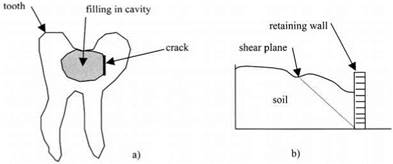
Similarly, the dentist does not rely on adhesion when he fills a tooth, but prefers to undercut the cavity, so that the filling is held by mechanical locking of the mercury amalgam or dental cement, as shown in Fig. 1.7. The tooth would thus have to break to release the filling material, which can become detached from the tooth by shrinkage, cracking, or chemical attack.
By the same argument, the designer of a retaining wall to hold back a soil mass does not presume that the soil has any internal adhesion between the particles, but instead designs the structure on the basis that the soil mass can slide freely on the plane shown by the dotted line. He knows by experience that the soil can have a considerable strength when dry. Dried clay and soil bricks are still
|
Figure 1.7. (a) Tooth showing undercut cavity, (b) Retaining wall holding back soil mass. |
widely used for building houses in developing countries. However, he also knows that, if the soil is wetted or changed in acidity, then the soil can be enormously weakened and can fail disastrously.
All these practices illustrate the lack of theoretical knowledge about adhesion over the centuries. Newton’s gravitational theory had suggested that universal forces existed holding masses together. Unfortunately, although gravitation can hold a rock onto the Earth’s surface, it is much too small a force to account for the observed values of adhesion between two cemented rocks. The next important step in understanding molecular adhesion forces arose from the kinetic theory of matter, which was developed towards the end of the 19th century. Strangely, like Newton’s laws of motion, this theory was developed on the crazy assumption that no adhesion existed between the atoms or molecules of a gas.
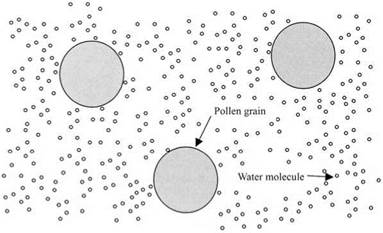
The need for a kinetic theory of matter arose from microscopic study of particles immersed in liquids.8 In 1827, a botanist called Brown had been observing, through his microscope, tiny pollen grains swimming in water. The water prevented the pollen grains from sticking together, giving a close approximation to zero adhesion. Brown could see that each grain of pollen was dancing in the watery suspension as though bombarded by invisible impacts, randomly hitting the particle from all directions. He concluded that the liquid was composed of very small atomic or molecular particles, too tiny to be visible in his microscope, in constant motion. The collisions of the invisible particles on the pollen grains were causing the dancing movement, as shown in Fig. 1.8.
|
Figure 1.8. Schematic picture of pollen grains immersed in water. The small spheres are water molecules which are 1000 times smaller than the pollen particles. All the particles are moving and colliding with each other incessantly. |
This was an amazing breakthrough in understanding the nature of molecules. It has still not been fully absorbed into our consciousness after two centuries. Most of us still believe that material is almost continuous and nearly static, whereas in reality it is always molecular and continually moving. This is the model of matter which led eventually to the theory of intermolecular adhesion. But the immediate effect of Brown’s observation was to stimulate theoretical argument about the properties of gases which, to a first approximation, behaved as though there was no adhesion between their constituent atoms. On this assumption, Clausius, Maxwell, Boltzmann, and their co-workers generated the mathematical theory describing the behavior of perfect gases.9 The speed of the moving molecules was then recognized to be the measure of temperature.
Van der Waals in 1873 was the first to consider that molecules must have attraction for each other, and that such attraction can be seen in a deviation from the perfect gas equations.9 This idea, that all bodies attract each other with a considerable adhesive force, was the beginning of a logical theory of adhesion, providing the first law, an idea which will be studied in much more detail in Chapters 3 and 5.
Van der Waals’ model explained how a gas would condense into a liquid or solid as a result of the attractive forces, once the temperature was reduced to slow the Brownian motion. This cooling of a material to form an adhering solid is one of the most powerful adhesive processes, that of thermoplastic adhesion. The idea of a “van der Waals” adhesive force accounting for the effects of adhesive processes has been a potent one, allowing the well-known empirical adhesive technologies to be explained to some extent.
 21 августа, 2015
21 августа, 2015  Pokraskin
Pokraskin 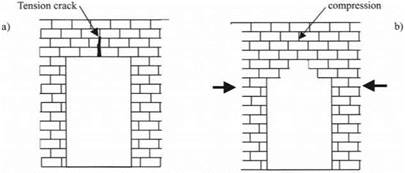
 Опубликовано в рубрике
Опубликовано в рубрике 