The laws of molecular adhesion are:
1. All atoms and molecules adhere with considerable force. More simply, if two solid bodies approach to nanometer separations, then they will jump
into contact as a result of molecular adhesion. This behavior differs from our ordinary engineering experience.
2. The effect of contaminant “wetting” molecules is to reduce adhesion, or even to make the bodies repel each other. In other words, adhesives reduce molecular adhesion.
3. Molecular adhesion forces are of such short range that various mechanisms can have large effects. Examples of such mechanisms are surface roughness, Brownian motion, cracking, viscous deformation, etc. These mechanisms lead to a rich variety of adhesion phenomena which may cause macroscopic adhesion to vary, even though the molecular adhesion remains the same.
These laws at first sight seem to go against our common experience. Indeed, they may seem to be incorrect when seen for the first time. Rather like Newton’s laws of motion, the above statements strip away the interference of other effects such as gravity, friction, viscosity and geometry which dominate our everyday experience. They reveal the chemical reality of natural electronic forces between atoms.
The best analogy is with Brownian motion.7 Before Robert Brown in 1827 observed the incessant spontaneous movement of pollen grains in water, it was believed that bodies were static. The breakthrough that Brown, and later Perrin, made was to recognize that this static appearance is false at the nanometer level. Molecular motion affects every particle in the universe. Whereas engineers had believed (and, in many cases, still believe) that objects stay put, we now know that all bodies in the cosmos are moving with an energy ЪкТ/2 where к is Boltzmann’s constant (1.4 x 10"23 JK~‘) and T is the absolute temperature. This idea of perpetual particle motion is quite foreign to most people, and engineers can usually ignore it without too much error, because the energy of movement is very small, around 10 77 J under ordinary conditions, too small to influence a car, for example, which would require perhaps 100 J of energy to give reasonable movement, 1022 times larger than the Brownian energy. However, designers of nanoscale machines must take this movement into account because objects below 100 nm in size are moving significantly.
Just as Perrin concluded that a fluid’s “apparent repose is merely an illusion” because the fluid molecules are in a state of eternal and spontaneous motion, so must we believe that all molecules adhere strongly, even though macroscopic objects appear nonsticky. The apparent lack of adhesion we see in engineering situations is really an illusion because adhesion is universal at the molecular level, according to the first law of adhesion above. However, there is a serious conundrum here because it seems impossible that particles can be in constant Brownian movement, where it is necessary for particles to collide and bounce off each other, yet also sticking together, which would cause agglomeration and
|
Figure 3.8. (a) Brownian picture of the universe with all particles moving, (b) The true picture has some particles adhering. |
ultimate static behavior. That is the problem addressed in Chapter 5. There has to be a balance between Brownian movement and sticking, as illustrated in Fig. 3.8. The conclusion is that, within a Brownian system of moving objects, there must always be some adhering particles. In other words, a Brownian system cannot be fully dispersed in reality.8
Here then is a mechanism, Brownian motion, which has an enormously strong influence on adhesion phenomena. There are many other mechanisms which must also be accounted for. For example, why do the pages of this book not stick together to form a solid cellulose mass. This is not a ridiculous question. Goodyear, the inventor of rubber crosslinking, was producing waterproof mail bags 180 years ago by coating them with rubber. Unfortunately, the sheets did stick together completely and Goodyear went bankrupt. By chemically crosslinking the rubber to make it more elastic, removing the tacky contact mechanism of the polymer, he was able to solve this adhesion problem.
The laws of adhesion have stripped away all the interfering mechanisms to get at the underlying reality. Now we must add the mechanisms back in one by one to understand the ultimate complex mechanics of adhesion phenomena.
 7 сентября, 2015
7 сентября, 2015  Pokraskin
Pokraskin 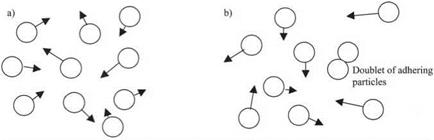
 Опубликовано в рубрике
Опубликовано в рубрике 