A simple way to distinguish molecular adhesion from electrostatic, gravitational, or liquid bridge adhesion is to observe the range of action of the force. Newton knew of the long-range action of those forces and was tantalized by the much shorter range of molecular adhesion.
The two most easily distinguishable forces of attraction are suction and liquid bridge adhesion. The suction pad, as explained in Fig. 2.5, relies on atmospheric pressure to hold the bodies together. This pressure remains constant as the pad is pulled away from its substrate. The same is true of a liquid droplet acting to glue two balls together; the force of adhesion is constant as the balls separate, as shown schematically in Fig. 2.12. Thus these two types of adhesion
|
|
are tough; the force stays high with distance and so a great deal of energy is required to pull the bodies apart. The energy is the area under the line.
Electrostatic adhesion is not so tough because now the force falls off with the square of distance from the centers. But the force is still of long range and can be measured with distance on a large scale. You can measure the distance with a meter ruler, for example. Newton’s gravitation force also follows this curve but the force is very small.
However, molecular adhesion is very different. This falls off in a very short distance of separation. As a consequence, these molecular adhesion forces cannot be measured with a meter ruler, but need a nanometer scale. The adhesion force may be high when the molecules are touching, but even a separation of one nanometer causes the force to drop almost to nothing. Thus the surfaces snap apart in a brittle fashion, totally different from the other types of adhesion force. The area under the curve is very small. In other words, the energy of molecular adhesion may be negligible. Taking this energy for one square metre of joint, we define the work of adhesion Win Joules per square metre.
The other feature of molecular forces which is evident from this comparison is seen when the balls are brought back together. The suction pad and the liquid drop make contact again very easily. Also the electrostatic bond is readily renewed as the balls touch again, to give the same strength as before. But the molecular adhesion is not easily regained. The smallest speck of dust, or contamination by a single layer of foreign molecules, can prevent the molecular
bonding. In other words, the surfaces cannot be replaced in exactly the same position to reinstate the original bond. That is the fundamental cause of the adhesion paradox discussed in Chapter 1. Molecular adhesion is not reliable or easily repeatable, because molecules cannot easily be put back in exactly the same position.
As Newton recognized, this short range of action of molecular forces has made the force measurement extremely difficult. Only in the past few years or so has it been possible to make the necessary measurements at the nanometer level to prove these ideas. Two problems have been overcome. The first one was the measurement technique. Optical methods, such as multiple beam interferometry and laser optical levers, have been developed to measure atomic distances. Piezoelectric actuators were invented to control nanomovements. The second issue was surface smoothness. Smooth surfaces of oxides such as mica, silica or alumina have been found. Also tiny smooth probes have been made on a large scale by electronic wafer fabrication routes. These new techniques have allowed a new and proper definition of molecular adhesion.
 2 сентября, 2015
2 сентября, 2015  Pokraskin
Pokraskin 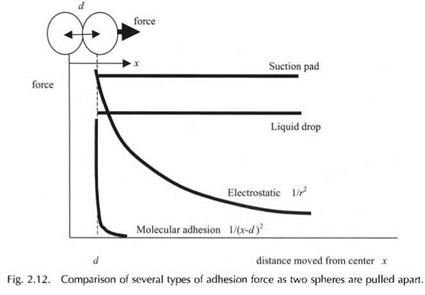
 Опубликовано в рубрике
Опубликовано в рубрике 