We normally observe adhesion at the macroscopic level, say by peeling a film from a surface as in Fig. 3.9. In order to understand the force which is necessary to pull the film off, we have to connect this mechanical picture with the molecular adhesion forces which we know to be universal at the nanometer level.
The macroscopic mechanism looks simple, because we grip the film and pull it off by applying a force (Fig. 3.9(a)). However, it is obvious that the applied force is not acting directly at the contact. The film has to bend and apply leverage to the adhesive region. So there is a complex mechanical system operating. To understand this we need mechanics. In particular, we apply the continuum mechanics theory in Chapter 14.
|
Figure 3.9. (a) Macroscopic adhesion test, (b) mechanism of cracking, (c) molecular adhesion. |

If one looks closer, using a microscope, at the point where the polymer film is detaching from the oxide surface, at the micrometer level (Fig. 3.9(b)), then it is evident that there is a crack traveling along the interface between the polymer and the glass. This crack is the mechanism by which the polymer material detaches from the surface. All brittle adhesive joints fail by cracking. This is a mechanism which involves elastic deformations and creation of new surfaces. It can be analyzed by the energy balance theory described in Chapter 7.
Looking even closer at the precise point of detachment of the polymer, we begin to resolve the oxide molecules in the substrate, vibrating but not bodily moving from their crystalline positions. By contrast, the polymer chains are moving bodily in a liquid-like motion, and the ends of the chains are continually and rapidly making contact with the oxide, then breaking away. So the molecular picture is one of Brownian movement, where the molecules only stick onto the surface periodically. Thus, an equilibrium of molecular adhesion can be established, such that the point of detachment is where the rate of making contact is equal to the rate of breaking. This is the kinetic theory of molecular adhesion which will be discussed in Chapter 5.
The conclusion from these arguments is that adhesion needs to be seen at three distinct levels. Adhesion is a molecular phenomenon, so we must first examine closely the molecular behavior. But it is not possible generally to apply forces directly to the molecules. The forces are usually acting through a mechanism, often a cracking mechanism. Cracks can easily be observed in adhesive joints and the detailed nature of the mechanisms have been established.9 Finally, the forces are applied to the crack through a particular system of mechanics, in this case a peeling system. The way in which the peeling is carried out can have a large influence on the adhesion forces observed, even though the molecular picture remains the same. Thus we need to study adhesion on three levels: from molecules, through mechanisms, to mechanics.
This overview explains why there have been enormous problems of communication between adhesion scientists in different applications. Engineers
tend to be concerned with the gross macroscopic mechanics and produce lengthy mathematical explanations which do not normally mention molecules. At the other extreme there are chemists who synthesize molecules which alter the adhesion at the atomic level. But these behave differently in the different macroscopic tests. In between are the material scientists who study the microscopic cracking behavior, desperately trying to reconcile the test protocols with the molecular reality. In this book, the objective is to study these three views of adhesion together, starting with molecules, going on to the mechanisms and finally understanding the mechanics.6 First, the molecular behavior becomes evident because molecules jump into contact.
 7 сентября, 2015
7 сентября, 2015  Pokraskin
Pokraskin 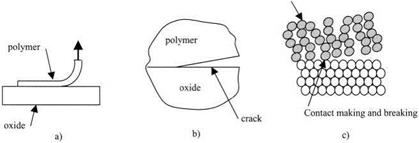
 Опубликовано в рубрике
Опубликовано в рубрике 