Many phenomena lead us to believe that molecular adhesion exists. We recognize that, just as a small solid particle sticks to a surface, so a droplet of water adheres to glass, and we realize, as Young and Laplace did in the early 1800s, that this can be explained by attractions between the water molecules and the glass. It is evident that this is an intensely local and short-range attraction, because changing the size of the droplet to make it very small does not influence the adhesion, as shown in Fig. 2.1.
Using liquids in this way, it is also very easy to show that the two major resisting effects mentioned in Chapter 1, surface contamination and roughness, are readily observable. Figure 2.1(b) shows that, when a thin, single layer of wax molecules is laid on the glass surface, then the water drop will not spread easily. Similarly, when the surface is roughened, the droplet is even more resistant to spreading. But it is evident that the roughness effect is different from the wax, which weakens both adhesion and detachment; the roughness weakens adhesion but strengthens detachment of the water from the glass. In other words it resists both processes of adhesion and detaching: it is hysteretic, in other words a process rather like viscosity in Newton’s theory of forces.
|
Fig. 2.1. (a) Water drops adhere to glass independent of droplet size, (b) Adhesion is reduced by both contamination and roughness. |
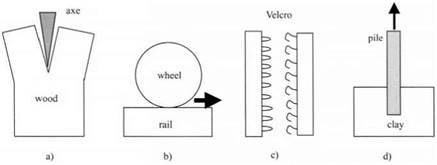
The problem with adhesion is that we often use the same word to describe quite different situations. For example, when we split a log with an axe, as in Fig. 2.2(a), we can see the cellulose fibers separating by fracture within the wood, and we conclude that the wood is made up of cellulose fibres strongly adhering together. This is a valid description.
However, when one describes the adhesion of a tire to a road surface2 or of a railway wheel to a steel rail, as in Fig. 2.2(b), this means something entirely different. In fact, there is no significant adhesion force in this case as one tries to lift the wheel from the track. Instead, the writer is referring to the force required to make the wheel skid along the surface. This phenomenon should be called friction and not adhesion. If the railway was built on the surface of the Moon, however, there could well be significant adhesion then between wheel and rail because the surfaces are much cleaner, giving improved contact. Friction would then also increase.
In another example, Velcro fabric or a zip fastener is said to produce adhesion between two pieces of material as shown in Fig. 2.2(c). But such devices operate on a hook and eye principle, in which the hooks of one piece becomes entangled with the loops of another. The separation of such Velcro joints requires friction to slide the fibers across each other, plus deformation of the curved hooks to obtain release. No adhesion between the materials is necessarily needed.
Similarly, a book on the “Adhesion of piles in stiff clays”3 is more about frictional sliding of the pile as it is pulled out of the clay, rather like the fiber pullout test described in Chapter 16. However, once the pile is removed, clay may then be seen adhering to the steel surface. Clearly, both friction and adhesion can be observed simultaneously in this case, as shown in Fig. 2.2(d).
The conclusion from this discussion is that word “adhesion” should be used only to describe those phenomena in which a normal force is needed to separate
|
Fig. 2.2. (a) Fracturing a wooden log with an axe; (b) sliding a wheel on a rail; (c) Velcro fabric made of hooks and eyes; (d) pulling a steel pile out of a clay bed. |
materials, e. g. by fracture, and not to describe frictional effects where sliding or shear is the force.
Friction is therefore different from adhesion. It is worth considering the differences in more detail because the friction problem is as old as that of adhesion,4 becoming known as tribology after the Greek word “tribos” meaning rubbing, during the celebrated studies of Bowden and Tabor.5 Friction had obviously been important in producing fire during ancient times, and preventing friction was valuable in construction and in chariot building, as shown by Egyptian wall-paintings which showed lubricant being poured in front of a moving stone slab about four thousand years ago.
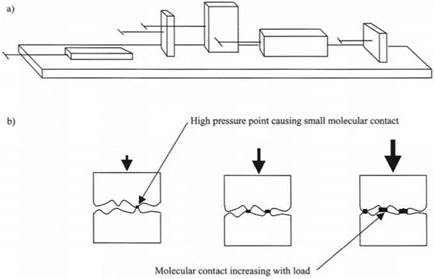
Friction was known to be similar to adhesion because it was affected by surface contamination and also by time of contact. Leonardo da Vinci had demonstrated the two laws of friction, which were rediscovered later by Amontons and Coulomb, and wrote in his notebooks Codex Atlanticus and Codex Arundel that “friction produces double the amount of effort if the weight be doubled” and “friction… will be… equal… although the contact may be of different breadth and length.” This last is extremely surprising at first glance and is illustrated by Leonardo’s diagram shown in Fig. 2.3. An engineer would surely expect the force to rise if the area of contact was larger. But this was not so for most cases.
The explanation of these old laws was put forward by Bowden and Tabor following Holm’s studies6 of electrical conduction through metal contacts. It was demonstrated by elegant theoretical and practical arguments that the molecular contact between two metal blocks was very much smaller than expected from the simple geometrical appearance. But as the load increased, the molecular contact increased in proportion to load, as shown in Fig. 2.3(b), because the pressure was so high at the small points of contact with the material flowed plastically. Thus the friction also rose proportionally to the normal load.
The difficulty with this theory was that no adhesion was usually observed after the normal load was removed. If the materials had been in molecular contact, causing cold-welding at the high pressure points, then surely some residual adhesion would be noticed when it came to separating the objects. The ingenious explanation put forward was that the elastic recovery of the materials would be sufficient to break the adhesive joints as the load was lifted. In fact, with clean metals, adhesion was generally observed, and transfer of metal fragments from one surface to the other could be detected, thus proving that strong adhesion had occurred in small localized contact regions.
|
Fig. 2.3. (a) Leonardo da Vinci’s sketch of friction experiments.4 (b) Bowden and Tabor’s explanation.5 |
 27 августа, 2015
27 августа, 2015  Pokraskin
Pokraskin 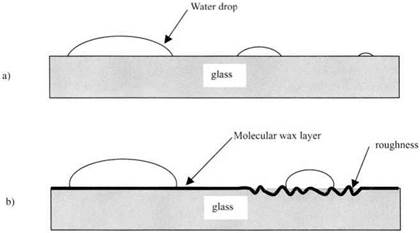
 Опубликовано в рубрике
Опубликовано в рубрике 