After examining the way in which a particle sticks to a surface, as above, it becomes apparent that adhesion is not a single process, but one which we can separate into three different but related actions: jumping into contact, achieving a certain black spot size, then cracking apart as a tensile force is applied.
The first adhesion phenomenon is the most convincing: all particles leap spontaneously onto surfaces, showing that the molecular attractions do not depend on any extraneous influences, such as adhesive molecules or surface keying. Thus adhesion can be measured by looking at the distance covered by the leap. A long jump means strong adhesion. This ingenious method of quantifying adhesion was first applied systematically by Tabor and Winterton in the late 1960s and will be described in detail in Chapter 4.
The second phenomenon, the achievement of a black spot resulting from adhesion forces, was the idea mentioned by Newton as a measure of true molecular contact, but this was not measured sensibly until 1971.3 This black spot should really be the equilibrium size which balances the molecular adhesion forces trying to enlarge the black spot, in competition with the elastic forces in the rubber trying to force the particles apart. This balance defines the size of adhesion. A large spot means large adhesion. Clearly, this is a dynamic equilibrium at the molecular level, even though the black spot seems to be static when viewed macroscopically with a magnifying glass.
The third phenomenon, that of detachment of the particles from each other by applying a pull-off force, is the test of adhesion which is most familiar to us. We increase the tension force applied to the particles until they just come apart, and define that force as the adhesion force. A large force means large adhesion. However, this is a very difficult experiment to carry out because the final detachment is an instability which is hard to reproduce exactly each time. Thus, many different values of adhesion can be found for the same samples in such tests, depending on the rate of loading, the precise moment of detachment, etc., leading to considerable unreliability in such measurements.
These three adhesion measures of molecular adhesion, in the contact make — and-break process, also allow us to test the second law of adhesion, that contaminant molecules always decrease the attraction between bodies. Consider immersing the rubber spheres in water and repeating the adhesion experiment. The results show that all three indicators of molecular adhesion: (the jumping into contact, the size of the contact spot, and the pull-off force) are diminished by the presence of the water molecules. Adhesives reduce adhesion!
When the rubber spheres are brought together under water, they can approach much closer before the jumping occurs. This suggests that the attractive force pulling the spheres together is reduced. Once the spheres have leaped into contact, the contact spot can be seen expanding to its equilibrium value. But now in wet conditions, the contact spot size is much smaller, indicating that the adhesion is less. Similarly, when the spheres are pulled apart under water, the force required is about ten times less than in dry conditions, showing a much reduced adhesion (see Fig. 3.15).
Thus it is evident that the presence of the water molecules on the rubber surfaces has diminished the molecular adhesion, in accordance with the second law. These reductions in adhesion produced by contaminants become obvious when studied in the atomic force microscope.
|
Figure 3.15. Comparison between black spot sizes for dry and wet spheres in perfect molecular contact. |
 12 сентября, 2015
12 сентября, 2015  Pokraskin
Pokraskin 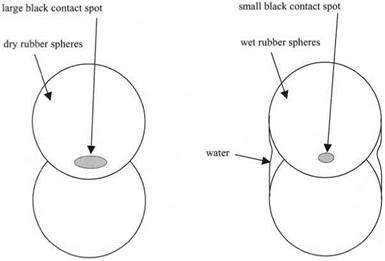
 Опубликовано в рубрике
Опубликовано в рубрике 