Long before people made their investigations into adhesion technologies, natural phenomena were occurring on the Earth’s surface, illustrating the processes of adhesion which we now understand to some degree.
For example, volcanic ash was deposited over many regions. This could cement together when wet to produce the porous rock which encapsulates the ruins of Pompeii, for example. Similarly, solid particles were deposited in seas and river estuaries. These adhered together under the influence of chemistry,
|
figure 1.11. Synthetic polymer latex particles. |
temperature, and pressure to form sandstones, mudstones and ultimately meta — morphic rocks. Sandstone-like products can be made synthetically by heating compacted sand at alkaline pH around 13 under 100 atmosphere pressure of steam.
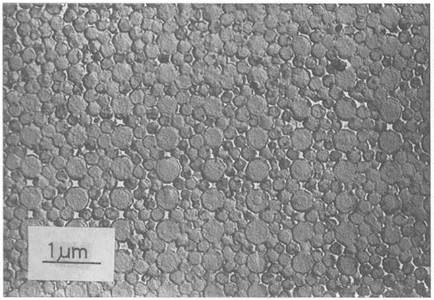
A peculiar type of silica rock which has obviously been formed by adhesion of small particles, is opal. Its structure was only seen in detail when it was inspected by electron microscopy in the 1960s by Saunders and Murray,15 as shown in Fig. 1.12. It became clear that the structure was regular at the 0.2 jam level, near the wavelength of light, because opals gave colorful light scattering patterns. Microscopy proved that this coloring effect was caused by the opaline structure of uniform spherical silica particles arranged in almost perfect order. It seems likely that the perfect spheres were precipitated from silicic acid solution in some ancient sea. Over a long time, the spheres would sediment together, causing the regular structure to appear. Then the water dried out to leave the spheres in contact, which would then give substantial adhesion by the effects of pressure, temperature, and water vapor. Synthetic opal can be made by this route.
Another example of the variation of adhesion in natural rocks is that of fossils. When rocks are formed by the adhesion of the fine particles of a mineral,
|
Figure 1.12. Electron microscope structure of opal |
any embedded creatures will influence the structure and give variations in the adhesive strength. Thus, when such a rock is fractured, the cleavage tends to follow the weak interface between the rock and the fossil, thus revealing the mark of ancient organisms in the Earth’s crust, as shown in Fig. 1.13.
However, living things provide the most interesting examples of adhering structures. The earliest fossils indicate that primitive living cells were sticking to rock surfaces to form clumps. These fossils are the stromatolites,16 some 2000
|
Figure 1.13. Fossil shells revealed by the imperfect adhesive interface in rocks. |
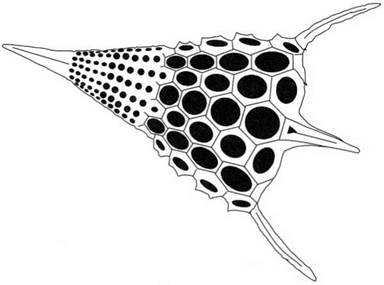
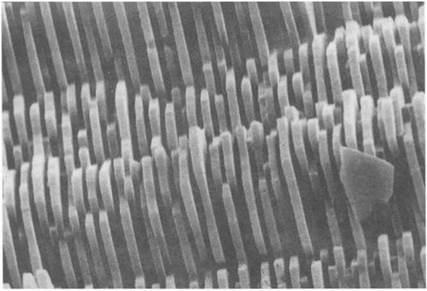
million years old, which were found in the siliceous chert rocks near Lake Superior. The algal cells could also secrete limestone, calcium carbonate, which could cement to form larger structures on which the living cells could proliferate. The huge coral reefs which abound in tropical seas show clearly the change in living matter which became possible when cells evolved beyond the slimy, filmforming stage to build substantial rocky structures from excreted minerals. In certain organisms, such as the diatoms, this adhesive growth has a fascinating beauty, as shown in Fig. 1.14. Extraordinary architectures are organized by the cells, somehow by templating the adhesion of the silica nanoparticles.
About 500 million years ago, the most successful adhesive skeleton structure evolved in the mollusc, with superb properties: mother of pearl (Fig. 1.15). There are about 60 000 species of mollusc living today, each one depositing a shell material for protection from predators. This shell is excreted from the organism, but builds up in an organized lamellar form, with each crystal of the shell separated from its neighbor by a thin film of protein. The structure of such kinds of mother of pearl has been linked with the remarkable toughness of the shells. They are a hundred times more crack-resistant than the inorganic limestone material itself.17 Some of these molluscs, like the limpet, also secrete protein to glue themselves to the rocks below. Many investigations have been carried out to determine the nature of this wonderful, water-resistant adhesive.
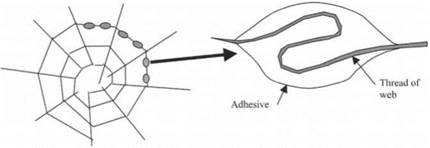
This secretion of adhesive glue reached its perfection in the spider, which evolved some 300 million years ago. Although the structure of spiders’ webs may
|
Figure 1.14. Diatom structure seen in scanning microscope. |
|
Figure 1.15. Structure of mother of pearl, showing the plates of calcium carbonate. |
look obvious, there are still questions about how the silk interacts with the watery adhesive droplets condensed on them (see Fig. 1.16). For example, in certain webs the silk is pulled into the droplets and this provides the silk with more give.18
Obviously, one of the most compelling reasons for studying adhesion is the fact that our bodies are grown and held together by these forces. From the moment that the sperm cell attaches itself to the egg, and the egg adheres to the wall of the womb, we live by the attractive forces of adhesion. Of course, we would end up as mere blobs of jelly without the strong adherence of the calcium
|
|
|
|
phosphate bone particles to build our skeleton. Our skin would be weak and soggy if platelets of polymer had not adhered sufficiently to provide elasticity. Our brains could not work if the nerve cells had not made adhering communicating contacts.
On the other hand, it is a blessing that our red blood cells do not stick together because capillary flow would then be impossible. Unfortunately, some cancer cells are not sticky enough and spread because the malignant cell adhesion is too low to prevent migration. The conclusion is that we wish to understand and control both high and low adhesion within our bodies.
 21 августа, 2015
21 августа, 2015  Pokraskin
Pokraskin 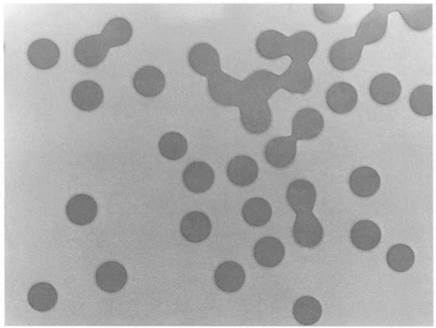
 Опубликовано в рубрике
Опубликовано в рубрике 