Contact angles between solids and liquids of known surface tension can provide an indirect measurement of solid surface free energy, allowing a comparison between different surfaces. Just as liquids have surface tensions or surface energy, so do solid surfaces by virtue of the fact that they are surfaces. Surface tension of solids goes unnoticed because they are usually too rigid to be visibly distorted by the interatomic, rather than intermolecular, forces holding them together.[1] Zisman(18) introduced the useful distinction between high energy surfaces and low energy surfaces. Most liquids have surface free energies below 100 mJ m"2 with organic adhesives having low surface free energies — usually 50 mJ m~2. Solid surfaces having similar free energies, such as plastics, are termed low energy surfaces. Hard solids, including the vast majority of metals and metal oxides, when atomically clean, have surface free energies typically in excess of 500 mJ m-2; these are termed high energy surfaces. Some values for surface free energies of interest are collected in Table 3.1. An energetic surface will make a wetting
|
Table 3.1. Values of surface free energies
|
|
High energy surfaces
tlarge polar component, •yf |
liquid spread on it, rather than remain as a discrete drop, so that adhesives should readily spread and wet the oxide layers of metallic substrates and the solid components of concrete (i. e. ySv > 7lv)-
The problem with high-energy adherend surfaces is that atmospheric contaminants are readily adsorbed on them, so reducing the surface free energy of attraction for the adhesive. Kinloch(2) suggests that the polar nature of structural adhesives will lead to displacement of the less polar, often hydrocarbon, contaminants. Polar water molecules, on the other hand, seem less likely to be readily displaced and, in view of their very small size in relation to an adhesive macromolecule, are able to adsorb in vast numbers on the surface.
Zisman(18) measured the critical surface tension, yc,[2] of a number of different metal and oxide surfaces which were exposed to atmospheres with controlled humidity. It was found that yc for all substrates was lowered to ~45 mJ m-2 at 0.6% r. h. and to ~37 mJ m-2 at 95% r. h. Gledhill et a/.(19) extended this work to the wettability of mild steel substrates of different surface rugosities and deduced the value of ySv as a function of relative humidity (Fig. 3.5). Further, the enhanced wetting of gritblasted steel at low humidities was reflected directly in higher joint strengths. Hence
|
"ySv (MJ/m2)
Fig. 3.5. Effect of humidity on surface free energy of steel (Ref. 19). |
any clean hydrophilic surface such as metal, metal oxide or the exposed aggregate in concrete is converted, upon exposure to a humid atmosphere, from a high energy surface to a low energy surface, with a surface free energy barely greater than that of the adhesive. These considerations demonstrate the importance of conducting the bonding operation in an environment which is as clean and dry as possible. Similarly by priming a substrate the surface free energy is reduced considerably so that the primer/adhes — ive system must be chemically compatible.
Mittal(12) reports that a direct relationship between VKA or yc and joint strength has been found to exist for some adhesive/substrate systems. The interfacial free energy is the most important surface property in that the lower the value of ySL, the greater the theoretical adhesion. However, for the same value of ySL different adhesives yield different experimentally measured bond strengths, leading to the conclusion that joint strength or ‘practical adhesion’ cannot be equated directly with the thermodynamic work of adhesion, VKA. Mittal states that this discrepancy is because (a) during joint rupture a number of inelastic deformations occur with consequent dissipation of energy, and (b) VKA refers to a defect-free interface, which is never the case. Other authors(2,20,21) have shown that the thermodynamic work of adhesion in water is negative for typical metal oxide/epoxy interfaces, indicating that water is capable of displacing adhesive from the substrate if no other forces are involved. Thus bonds to high-energy polar adherends are theoretically unstable in the presence of water, and this is borne out by experience.
 16 июля, 2015
16 июля, 2015  Malyar
Malyar 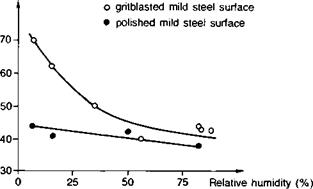
 Опубликовано в рубрике
Опубликовано в рубрике 