In general, adhesive application to painted surfaces is not to be recommended. However, just as the correct surface treatment is necessary, the application of an adhesive-compatible primer coating may also be desirable. Naturally, the picture of the adhesive-adherend interfacial zone then becomes more complicated. The use of adhesive primers may be more critical in some instances than others but often the advantages to be gained, especially ‘prewetting’ of the substrate surface, far outweigh possible disadvantages such as an extra process, or the primer or a primer interface becoming the weakest link in the joint. The experience of paint and adhesion technologists is that primers greatly reduce the variability of subsequent interfacial bond performance, and that certain products can create a water-stable interface. Their use may also obviate the need for complex surface pretreatment procedures.
As with adhesives, so with primers. The number of candidate products is enormous in order to fulfil any combination of the following requirements:
(1) The immediate coating of a newly prepared surface protects it from damage and contamination. The high surface energies of metals and aggregates are thus converted to ones of much lower surface energy, albeit highly compatible with the adhesive used.
(2) The opportunity to wet the surface more easily and thoroughly than the high viscosity of the adhesive itself would allow, thus facilitating penetration of the surface irregularities and oxide layers.
(3) The ability to block the pores of a porous surface and so prevent capillary suction of adhesive away from the bondline. For concrete such ‘bond coats’ are often employed, the resin being applied whilst this layer is still tacky.
(4) The provision of an improved mechanical key. In cementitious repair work the ‘bond coat’, while still tacky, may be dry — dashed with sharp sand.
(5) Corrosion inhibition, which implies treatment of the metallic substrate surface.
(6) Sacrificial pretreatment, by acting as a hydrophobic ‘preferred contaminant’ to enable bonding underwater(70).
(7) The possibility of avoiding the need for complex pretreatments by promoting chemical bonding with coupling agents.
(8) Prevention of the displacement of the adhesive from the substrate surface by water if chemical bonds can be formed with coupling agents and hydrosols.
In fulfilment of these functions, the two basic approaches which may be discerned are (a) providing a relatively thick barrier or surface coating, and (b) applying a coupling agent to the substrate surface as a monolayer; Fig. 3.13 illustrates the difference. Conventional primers are composed of dilute solutions, 10% solids or less, of the adhesion resin itself in an organic solvent or blend of solvents. Additionally, the primer may incorporate agents to assist wetting, flow control, curing, inhibit corrosion, and toughen the cured primer layer. Light abrasion and solvent degreasing of the primed surface is often advised before application of the adhesive. Hewlett(67) advocates the use of penetrating sealers and primers for concrete surfaces, whilst Hugenschmidt(71) recommends an epoxypolyurethane coating with a zinc-chromate base for priming steel plates to be used as externally bonded reinforcement in Switzerland. The danger of relatively thick primer layers is that they may become the weak link in a joint because they are themselves mechanically weak. The alternative approach is the use of coupling agents which are used, not specifically to improve bond strength when dry, but to enhance environmental durability. The most common are the siloxanes(72-74) and titanates(75-77), which are applied to metallic or siliceous surfaces in aqueous solutions as a monomer.
Siloxane or silane coupling agents are so termed because they possess a dual reactivity, and are hybrids of silica and of organic materials related to resins. There remains, however, some debate as to the factors affecting the mechanisms by which these materials
|
Fig. 3.13. Primers and coupling agents. 106 |
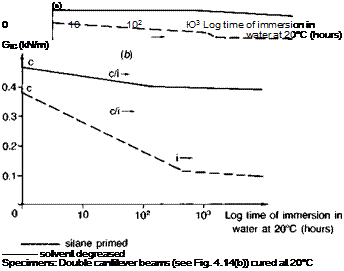
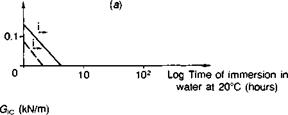
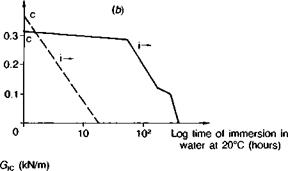
function(73). Basically, when applied to metal or glass surfaces as a monomer a condensation reaction occurs as it is dried on, leaving a very thin resinous layer which is attached through primary (silicon-oxygen) valency groups to the metal oxide or glass structure. The high energy substrate surface is now hidden and replaced by a surface of relatively low energy. Water displacement of this coating is unlikely since hydrolysis of the silicon-oxygen linkage is a slow process, requiring excess water to be present. In practice, a chemical connection is built into the other end of the silane molecule by inserting organic groups, such as amino-, or epoxy-, which are functionally reactive towards features of the adhesive molecule. It is because of the existence of a truly chemical connection, between oxide surface and siloxane on the one hand and siloxane and adhesive on the other, that these materials are known as coupling agents. Such materials may also be incorporated in the adhesive itself to promote interfacial bonding both with the bulk matrix (resin-filler) and at the adhesive-substrate interface. In GRP technology silanes are used as a matter of course, because water will displace resin from glass; there would otherwise be no fibreglass boats or warships. Comyn(78) cites the case of glass tiles, bonded with epoxy, falling off a London hotel and this problem was subsequently remedied by the application of a silane primer. Figs. 3.14 and 3.15 indicate the superior environmental stability conferred on steel surfaces; when applied to gritblasted steel adherends the interface was found to be completely water-stable.
Gettings and Kinloch(23) used surface-specific techniques to ascertain the bonding mechanisms between silane primer and mild steel, and established that a 1% aqueous solution of Union Carbide’s A187 dramatically enhanced joint durability. Hewlett and Pollard(17) examined coupling agents in connection with silicate materials and injection resins, as described earlier, and Allen and Stevens(79) employed infra-red spectroscopy to elucidate the structure of siloxane coupling agents on aluminium. Walker(80), in a series of articles, reported on tests involving a number of silanes, applied to various metallic surfaces and incorporated in a range of paints and coatings. In their application, the four variables to be addressed for any one choice of siloxane are (a) weight of addition (b) composition and pH of the alcohol/water solution (c) time allowed for the mixture to stand before application and (d) time elapsed before application of the adhesive. Due to their hydrophobicity, silane primers are increasingly being used as one of a range of concrete surface ‘sealers’,
|
|
|
|
|
|
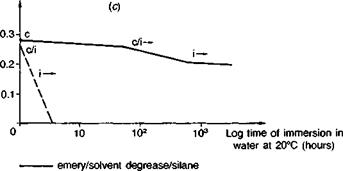
———- emery/solvent degrease
Specimens: steel wedge cleavage (See Fig. 4.14(c)) cured at 20°C
c: cohesive crack growth i: interfacial crack growth
Fig. 3.14. Performance of emery cloth abraded adherends, and the influence of a silane primer, in stressed cleavage tests (Ref. 4). (a) Aromatic amine cured epoxy. (b) Aliphatic amine cured epoxy, (c) Epoxy polysulphide.
 |
c: cohesive crack growth i: interfacial crack growth
Fig. 3.15. Effect of silane priming of gritblasted adherends prior to bonding in stressed cleavage tests (Ref. 4). (a) Aromatic amine cured epoxy. (b) Aliphatic amine cured epoxy.
particularly as applied to bridge decks(81,82). The mechanism by which they work is to make the surface cement pores water-repellant, so that salt solutions are not drawn into the body of the concrete by capillary action.
One of the most promising innovations in recent years is the development of hydrophobic Sacrificial Pretreatment Technology (SPT), in conjunction with hydrophobic cold-cure epoxies, to enable underwater bonding(70,83). The energetically-favourable conditions for bonding are established underwater by the application of a water-repellant ‘preferred contaminant’ to a cleaned or blasted steel
|
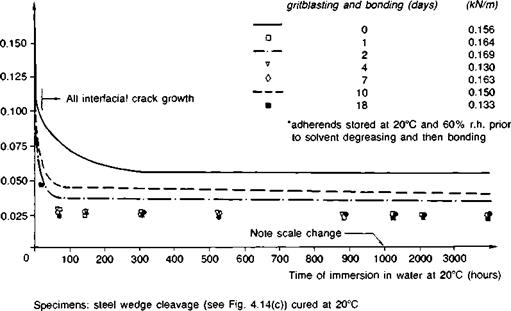
Fig. 3.16. Effect of time lapse between gritblasting and bonding in stressed cleavage specimens constructed with an aromatic amine cold-cured epoxide (Ref. 4). |
surface. The adhesive, when applied over this compatible film, either absorbs or displaces it, adheres to the substrate and cures to give a hydrophobic polymer matrix. Burns(84) discusses an equally interesting method of repairing weld failure in oil storage tank floating roof seams, by using an oil and water tolerant cold-curing sealant. A new method of enhancing and maintaining the adhesion between epoxies and steel consists of a process of organic modification of a thin layer (about 10 nm) of tin hydrosol particles, previously deposited onto the substrate from a wetting hydrosol dispersion(85). Corrosion protection and wet peel strengths achieved were superior to all other surface treatments including etching, phosphating and silane application.
 23 июля, 2015
23 июля, 2015  Malyar
Malyar 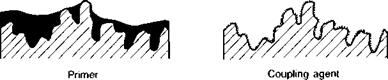
 Опубликовано в рубрике
Опубликовано в рубрике 