Metal and oxide surfaces. In joints involving metallic substrates, the adhesive ‘sticks’ to the metal surface oxide layer and not to the metal itself. Such joints can cause problems in service because oxide structures, and bonds to them, are susceptible to environmental attack.
Refined metals, by their nature, are chemically unstable, tending to react with their environments and reverting on their surface to metallic compounds probably very similar to the minerals from which they were originally extracted. The most common such reaction is with oxygen, giving rise to a surface oxide layer, and the rate of reaction increases with increasing temperature. The higher the temperature, the thicker the oxide layer, and the more difficult it is to obtain satisfactory wetting by an ‘adhesive’, be it molten metal or an organic polymer. Because of the very high temperatures involved in fusion welding, the rapid surface oxidation necessitates very strict control of the process to achieve adequate bond or fusion. Hence most high alloy metals which oxidise rapidly, such as stainless steel and aluminium alloy, are particularly awkward to weld or to bond.
Environmentally stable metals with protective surface oxide layers are generally unsuitable for bonding without pretreatment because their oxide structures are mechanically weak and up to 3000 nm thick. These must be replaced by stronger, coherent and stable oxide structures. Conversely, iron and plain carbon steel require little surface treatment, provided their surfaces are free from rust and millscale, because the oxide layer (Fe203) is only about 3 nm thick. Under normal ambient conditions, the outermost surface oxygen groups hydrate, albeit much more slowly than the surface oxidises, to form a high density of hydroxyl groups. This surface then adsorbs several molecular layers of bound water which, for metallic and siliceous surfaces, are retained up to about 400 °С. It is these hydrated polar groups which form bonds with the polar organic resins(14,38), the relevant adhesion forces being dispersion and hydrogen bonds.
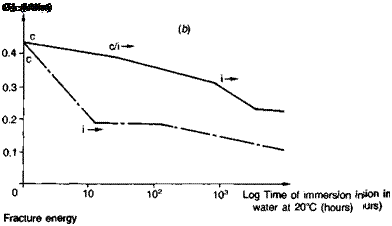
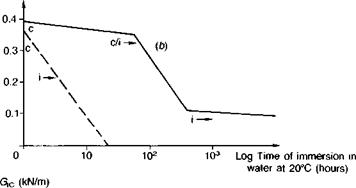
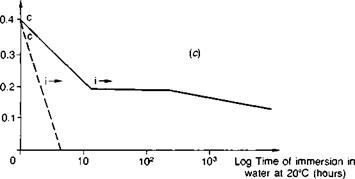
Steel. Publications specifically about steel surface preparation are by Sykes(39), Haigh(40) and Brockmann(41). Abrasive treatment is on the whole best for preparing plain-carbon steel, and any obvious rust or millscale should first be removed by wire brushing followed by degreasing. Sykes cautions that surfaces rusted in industrial or marine atmospheres will be contaminated by ferrous sulphate or chloride, and crystals of these salts are hard to dislodge even with blasting. Further treatment involving simple abrasion with emery cloth or wire brushes merely scores the surface, tending to rub contamination into the grooves which then becomes hard to dislodge; there is insufficient surface cutting action to encourage wetting by cold-cure adhesives. Brockmann(41), for instance, reports on the influence of the steel surface condition on which a one-part epoxy was heat-cured. By subsequently etching away the metal, then only in the case of shotblasted steel was a true replica of the steel surface morphology observed; the adhesive was found to have a porous texture when cured against ground — or degreased-only surfaces, indicating non-wetting. Figure 3.8 illustrates the very poor performance of emery abraded surfaces, with the specimen joints splitting apart completely along the adhesive-adherend interface within a matter of hours. Silane priming of the substrate surfaces was found to delay the time to failure, being particularly beneficial in combination with an epoxy polysulphide adhesive (see Fig. 3.14).
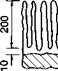
Shotblasting procedures, commonly employing angular chilled iron grit in the construction industry, remove inactive oxide and hydroxide layers by cutting and deformation of the base material leading to a fissured surface topography (Fig. 3.7). For other metals, alumina and carborundum are preferable hard, sharp abrasives. Shields(29) warns against the use of glass or metal beads of round shape as leading to peening of the surface. The size and nature of the abrasive grit should be matched to each type of metal and alloy, and different metal grits will leave traces of different metals on the blasted surface. Further, the geometry of the blasting nozzle and the pressure of blasting will affect the resultant topography. It is important to degrease the surface before abrasion, and to ensure that the grit particles are themselves free from contamination. Abrasive dust should then ideally be removed from the surface prior to bonding (a vacuum head fitted with edge brushes is recommended for structural steelwork(40)). Solvent degreasing, unless closed vapour systems are employed, is inadvisable because any contamination may simply be redistributed; there may also be other subtle undesirable surface effects. Figure 3.9 illustrates the deleterious effect of solvent degreasing following blasting. The use of wet blasting is limited in its applicability to non-corrosive metals unless anti-corrosion additives are included in the water; stainless steels can be blasted wet or dry with non-ferrous particles such as alumina, garnet or silica. Assessments of the surface finish of blast-cleaned steel for painting are covered by BS 4232(43) and SIS 05 59 00(44).
Chemical treatment of ferrous alloys with sulphuric or hydrofluoric acid, is not straightforward because of the precipitation of free carbon on the surface known as ‘smutting’, and the consequent need for ‘de-smutting’ immediately after etching(45). The metal then needs to be rinsed and washed in running water before transference
|
Fracture energy G|C (kN/m)
|
|
|
|
|
—— gritblasted emery cloth abraded
Specimens: steel wedge cleavage (see Fig. 4.14(c)) cured at 20°C
c: cohesive crack growth i: interfacial crack growth
Fig. 3.8. Influence of surface abrasion on the fracture energies of bonded joints as a function of time of water immersion: gritblasting versus emery — cloth abrasion, (a) Aromatic amine cured epoxy. (b) Aliphatic amine cured epoxy, (c) Epoxy polysulphide.
 |
————— degreased and gritblasted
————— degreased, gritblasted and degreased again
Specimens: steel wedge cleavage (see Fig. 4.14(c)) cured at 20°C
c: cohesive crack growth i: interfacial crack growth
Fig. 3.9. Effect of solvent degreasing after gritblasting on the fracture energies of bonded joints as a function of time of water immersion (Ref. 42). (a) Aliphatic amine cured epoxy, (b) Epoxy polysulphide.
to a bath of isopropanol. It is, therefore, impossible thoroughly to clean and etch mild steel and to finish with a washing process without corrosion occurring. The temperature and duration of each of the operations are crucial to the resultant surface morphology, and to complete ‘de-smutting’. Priming is advised as soon as possible after the final washing procedure. Trawinski(46) reports on a simple chemical process for plain carbon steel employing a nitric-phosphoric acid etchant, which is suitable for use at 21-27°C, and at remote locations. It is claimed to produce a smut-free microscopically rough surface, similar to that obtained with high temperature phosphoric acid-based etchants but with much safer resultant chemical residues. Further, superior performance was found in wedge cleavage tests over gritblasted and the usual phosphoric acid etch surface treatments, with a heat-cured epoxy and with or without primers.
The general term stainless steel is used not only for iron-nickel-chromium alloys of particular compositions, but often for any high alloy steels which are able to resist rusting in the presence of oxygen. The distinction between these classes is based on the types of (chromic) oxide film which form on their surfaces, and which retards the diffusion of aggresive ions. Oxygen (or an oxidising agent) is needed both to create this passive layer and to repair it in service; without it, stainless steel actively corrodes. Unfortunately, there is little published information on the effect of metal composition either on adhesive bond strength or interaction with etchant solutions. An acid etch pretreatment followed by ‘de — smutting’ and then priming is recommended: the number of etching procedures employed industrially are many and various. Gettings and Kinloch(24) found that the physical and chemical characteristics of the surface were strongly dependent on the manufacturing path of the steel, and that the environmental resistance of heat-cured epoxy joints was influenced both by the surface chemistry and the micro-roughness induced by chemical treatment.
Galvanised surfaces are characterised by relatively weak zinc oxide which may need to be removed by mechanical or, preferably, chemical means; a number of electrodeposition(35) and etch processes are described(47). Lees(48) cautions that although successful pretreatment is possible, the zinc layer itself remains as a potential source of weakness, since it might be stripped off by a good adhesive. Adams and Wake(45) further note that certain adhesives and anhydride-cured epoxies may form soaps at the zinc-adhesive interface. Nevertheless, for only moderately demanding situations simple abrasion techniques may suffice, depending upon the nature of the galvanizing processes and the resultant surface.
Some comparisons of the effect of surface pretreatment on mechanical joint strength, or measurable adhesion, are reproduced in Tables 3.5-3.7. It is stressed again that the important consideration is the effect on long-term bond integrity, and not on short-term strength.
Aluminium. Since the Second World War, aluminium and its alloys have been extensively investigated in connection with aircraft industry requirements, namely for joints of the highest strength and greatest durability. Mechanical treatment and alkaline cleaning on their own result in poor durability; chemical etching and acid anodising are favoured, and rigorous pretreatment processes are documented for deoxidising and allowing a controlled re-oxidation of the substrate surfaces with powerful oxidising agents. The gradual replacement of chemical etching by anodising follows the discovery that the nature and thickness of the re-formed oxide surface depends as much on the wash which follows as on the etch itself(45).
In addition to the brief description of pretreatment methods in the general texts(26,29-32) are many specific publications^,50-57). Contemporary opinion suggests that the function of pretreatment should be to produce a thick porous coherent oxide honeycomb, which imparts a degree of micro-mechanical interlock with the adhesive, and which is resistant to hydration. Hydration resistance and micro-mechanical interlocking have been the subjects of intensive study in recent years. A chromic-sulphuric acid etch has been favoured in Europe, whilst in the USA the Forest Products Laboratory (FPL) etch, based on sulphuric acid/sodium dichromate, has been optimised. The FPL etch is used alone or is followed by the Phosphoric Acid Anodising (PAA) process developed by Boeing. It has gradually been found that whilst etched adherends give higher initial bond strengths than do anodised ones, the latter confer more durable bonds. An explanation for this is that the oxide layer formed on etched adherends thickens with new, less coherent, oxide in the presence of moisture reaching the interface. Brockmann(3) suggests that the conventional phenolic resins confer an acid environment on the oxide surfaces, and it is this acidity which is inherently water — stable. Schematic sections through typical oxide morphologies are depicted in Fig. 3.10.
The choice of treatments for aluminium and its alloys revolves
|
Table 3.5. Effect of pretreatment on lap shear strength of steel! polyvinyl — formal-phenolic adhesive joints
Source: After Refs. 39, 49 and reproduced by kind permission of Elsevier Applied Science Publishers Ltd. |
|
Lap shear strength (MN m-2) After 30 days: water Initial immersion at 40 °С |
||||
|
Surface treatment |
23 °С |
80 °С |
23 °С |
80 °С |
|
Degrease in trichloroethylene. |
20.9 |
20.0 |
14.7 |
17.5 |
|
Degrease, light grit blast (alumina grit), degrease. |
24.8 |
31.4 |
16.0 |
18.3 |
|
Degrease, heavy grit blast, degrease. |
26.3 |
28.6 |
13.2 |
16.2 |
|
Etch in 100 g litre-1 sulphuric acid, 100 g litre-1 oxalic acid, (15 min, 90 °С), desmut by brushing. |
26.2 |
28.9 |
15.1 |
21.7 |
|
Etch in sulphuric acid/oxalic acid as above, desmut in sulphuric acid/chromic acid. |
27.3 |
33.9 |
21.7 |
28.8 |
|
Table 3.6. Effect of surface treatment of stainless steel on bond strength (EN58B steel-AV1566GB one component heat-cured epoxy (Ciba-Giegy)) |
|
Source: After Refs. 39, 49 and reproduced by kind permission of Elsevier Applied Science Publishers Ltd. |
 |
![]()
Fig. 3.10. Oxide morphology on aluminium-alloy after pretreatment (Ref. 58).
around the scale of operations, the metal composition, the adhesive to be used, the required durability, and the cost; thus there is a need for multiple recommendations. For example, for the potentially large-scale usage of aluminium in motor vehicle assembly Alcan International have developed an anodising process which is applied at the metal coil stage; the sheet material is then coated with lubricant for coil storage prior to pressing and then bonding.
Table 3.7. Effects of pretreatment for steel on bond strength and water resistance (AV1566GB one-component heat-cured epoxy (Ciba-Geigy))
Lap shear strength (MN m 2)
After 30 days:
water
immersion at Initial 40 °С
|
Material |
23 °С |
80 °С |
23 °С |
80 °С |
|
|
EN58B |
degrease |
23.9 |
25.6 |
15.7 |
17.7 |
|
stainless steel |
grit blast |
25.6 |
32.0 |
14.1 |
16.7 |
|
etch* |
27.4 |
35.1 |
27.2 |
29.7 |
|
|
EN58J |
degrease |
27.8 |
31.9 |
16.7 |
17.0 |
|
stainless steel |
grit blast |
27.3 |
34.0 |
29.0 |
25.0 |
|
etch* |
27.8 |
39.9 |
30.1 |
33.6 |
|
|
EN3B |
degrease |
20.4 |
24.3 |
9.8 |
7.3 |
|
mild steel |
grit blast |
23.7 |
27.4 |
15.9 |
18.3 |
|
*5 mm etch at 60 °С in 570 g litre’1 sulphuric acid, 100 g litre’1 oxalic acid. Source: After refs. 39, 49 and reproduced by kind permission of Elsevier Applied Science Publishers Ltd. |
A comparison of the effects of many different procedures is given by Poole and Watts(59), but Fig. 3.11 illustrates the general trend in performance. New processes, developed from the present understanding of adhesion, revolve around maximising micromechanical interlocking with the adhesive by ‘whisker reinforcement’, inhibiting oxide hydration by the use of phosphonate complexes(56), or both. For applications less demanding than those found in the aircraft industry it is possible to achieve reasonable levels of adhesion and durability by gritblasting with alumina followed by the rapid application of a primer or, preferably, a (silane) coupling agent.
 21 июля, 2015
21 июля, 2015  Malyar
Malyar 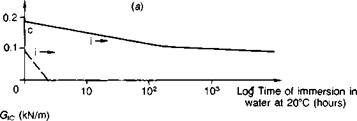
 Опубликовано в рубрике
Опубликовано в рубрике 