Whereas in epoxies the ‘hooks’ are provided by the resin and the ‘eyes’ by the hardener, in polyesters both ‘hooks’ and ‘eyes’ are located in the unsaturated resin(9). The addition of a curing agent or catalyst based on organic peroxide initiates the chemical reaction and promotes cross-linking within the resin. Unsaturated acids are
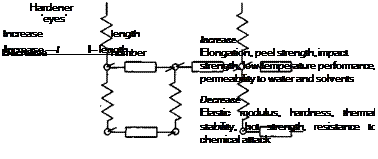 |
Fig. 2.4. Diagrammatic model of a thermoset adhesive (Ref. 9).
thus cross-linked by interpolymerisation with styrene to produce the so-called ‘unsaturated polyesters’ used as the basis of glass reinforced plastics and adhesives for constructional purposes.
Unfortunately, with polyester resins the contraction during cure can be as high as 10% by volume. This is partly due to the high level of thermal contraction which, unlike epoxies, tends to occur after the resin has set and can result in stress build up, possibly accompanied by interfacial cracking, at the resin/substrate boundary. In addition, there is a volume change during the transition from the uncured liquid phase to the hardened resin resulting in further curing shrinkage, in some cases due to solvent loss. Thus, there are usually strict limitations on the volume of materials that can be mixed and applied at any one time.
Reservations have also been expressed regarding the suitability of polyesters as structural adhesives because of the poor creep
resistance under sustained load and their bonding efficiency in damp or wet conditions, particularly to alkaline substrates. Nevertheless, they may be useful materials if a very rapid gain in strength is required from a material with a reasonable usable life after mixing. Several formulations also have the advantage of being able to cure in sub-zero temperatures.
 4 июля, 2015
4 июля, 2015  Malyar
Malyar  Опубликовано в рубрике
Опубликовано в рубрике 