The wide range of techniques for studying pretreatment effects may be classified as (a) mechanical (destructive) and (b) surface analytical (non-destructive). Naturally, different substrate materials warrant different approaches.
Experimental assessments of the effects of surface treatments using mechanical tests are of limited value unless environmental exposure is included. Appropriate comparative tests subject the interface to tensile, peel or cleavage forces but unfortunately most of these, including the pull-off test for adhesion(86,87), employ joints in which adhesive and adherend strains are far from uniform so that the results reflect an interaction of stress concentrations. Further, few of the joint configurations allow environmental access within a reasonable time-scale of exposure. However, the self-stressed fracture mechanical cleavage specimens described in Chapter 4 (4.14(band c)), and employed in most of the experimental work referred to in this chapter, do afford a quantitative means of assessing pretreatments for metals and the effects of environmental exposure in terms of measurable adhesion (2,4,5,88). The adherends are forced apart and fracture is calculated from a knowledge of the adherend displacement, and the crack length measured by travelling microscope. The location of the crack-tip is very important, because a crack will transfer rapidly from the bulk adhesive to an unstable interface. Initial crack propagation is generally rapid initially, and then stabilises to indicate a threshold fracture energy for the particular environment of exposure.
 |
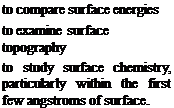 |
There is a wide variety of techniques available( 10,89):
Contact angle measurements, as discussed earlier, are limited in their applicability to ideal surfaces or to surfaces wetted by liquids of homogeneous composition resting in equilibrium. The nature of real surfaces, real adhesives and the pressure of bonding cannot be allowed for as encountered in real bonding operations. Nevertheless, some comparative information on surface energies and the wetting of some real surfaces may be anticipated.
Surface topography and morphology may be studied with optical or electron (scanning or transmission) microscopy, and these techniques are very useful for monitoring the physical changes due to pretreatments (e. g. Fig. 3.7). Indeed the significance of micromechanical adherend-adhesive interlocking has only recently been appreciated as an important mechanism of adhesion for bonds to certain adherends. Transmission Electron Microscopy (ТЕМ) gives even greater magnification than Scanning Electron Microscopy (SEM), but the former technique involves the preparation of replicas.
From many potential techniques to study surface chemistry, only a few are of particular importance. For metals Auger Electron Spectroscopy (AES), Electron Spectroscopy for Chemical Analysis (ESCA) also known as X-ray Photoelectron Spectroscopy (XPS), and Secondary Ion Mass Spectroscopy (SIMS) give useful information about the chemistry within the first nanometre of surface. ESC A (or XPS) is also used in the study of polymer surfaces, as are infrared techniques such as Multiple Internal Reflection IR and Fourier Transform IR. Inelastic Electron Tunnelling Spectroscopy (IETS) is a way of obtaining the vibrational spectra of molecules adsorbed on a metal oxide, and the technique has been used to examine adhesives and coupling agents. Good evidence of primary chemical bonds between certain silane coupling agents and both substrate and epoxide groups has been found(90,91).
 25 июля, 2015
25 июля, 2015  Malyar
Malyar  Опубликовано в рубрике
Опубликовано в рубрике 