The basic requirements for good adhesion are very simple, viz:
(1) intimate contact between adhesive and substrate
(2) absence of weak layers or contamination at the interface.
When two materials are bonded the resultant composite has several constituents and interfaces, as depicted for example in Fig. 3.2. Being liquid, adhesives flow over and into the surface irregularities of a solid, so coming into intimate contact with it and, as a result, interatomic forces are brought into play. Adhesives therefore join materials primarily by attaching to their surfaces within a layer of molecular dimensions, i. e. of the order of 0.1-0.5 nm. In joints involving metallic or siliceous substrates, the adhesive sticks to the surface oxide layer and not to the solid itself. In simple terms, there is an obvious conflict between having an adhesive material which
|
 |
|
|
||
|
||
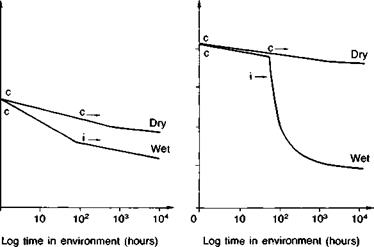
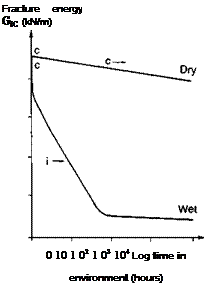
Dry: exposure at 20°C and 60% r. h. c: cohesive crack growth
Wet: immersion in fresh water at 20°C i: interfacial crack growth
Adherends: gritblasted bright steel bar
Specimens: double cantilever beams (see Fig. 4.14 (b)) cured at 20°C
(a) (b) (c)
Fig. 3.1. Effect of water on the fracture energies of bonded joints, as a function of time, (a) Aromatic amine cured epoxy, (b) Aliphatic amine cured epoxy, (c) Epoxy polysulphide.
spreads and adheres well to the substrate, and one which when cured is a highly cross-linked structure possessing significant cohesive strength. Elevated temperature curing provides one solution to this dilemma since viscosity will initially be lowered, so facilitating the flow of the material over the surface together with increased molecular mobility. In cold-cure products the presence of mobile mono-amines greatly enhances the wetting potential.
Adhesion is often discussed in relation to the strength of joints, but the force required to fracture a joint is resisted by a complex interaction of internally generated bondline stresses. Attempts to use joint strengths as a measure of adhesion can therefore be extremely misleading. In the ensuing discussion, the term adhesion is reserved for bonding across interfaces, and there are many useful recent publications on the science of adhesion(6-15).
 11 июля, 2015
11 июля, 2015  Malyar
Malyar  Опубликовано в рубрике
Опубликовано в рубрике 