Fillers. In practice most epoxy resin systems have fillers incorporated, often simply to reduce cost although they may also assist in gap filling, reduction of creep, reduction of exotherm, corrosion inhibition and fire retardation. Their incorporation will also alter the physical and mechanical properties of the adhesive. Construction resins in particular often include a large volume fraction of sand or silica.
In general, fillers are inert materials which may be organic or inorganic in nature. They increase the viscosity of the freshly mixed system but some also provide a shear rate dependency referred to as thixotropy. This is particularly useful for adhesives which are to be applied to vertical surfaces so that run-off can be minimised. The addition of fillers also serves to reduce the exotherm and subsequent thermal shrinkage on cure, and to extend the pot life. Thermal expansion coefficients are also lowered. With particulate fillers tensile and flexural strengths are usually reduced but compressive strength is improved. However, fibrous fillers can improve tensile strength and impact strengths. The effect on moisture and chemical resistance is less clear, there being some evidence(27) that the presence of fillers can cause a wicking action aiding ingress of moisture. However, plate-like filler particles may serve to reduce water transport. Traditionally asbestos was commonly used as a filler but for health and safety reasons this has now been replaced by materials such as silica flour, talc and aluminium powder.
Diluents. These are generally incorporated to reduce the viscosity of the freshly mixed adhesive to offset the effect of the filler. This may be required to improve handling and spreading characteristics or to allow filler additions which tend to reduce cost. Other properties of the fresh and hardened adhesive can be affected by the use of diluents, for example pot life, flexibility and glass transition temperature. If the diluent is non-reactive, such as solvents which remain in the cured system, the net result is a deterioration of chemical and mechanical properties such as increased shrinkage and reduced adhesion. Reactive diluents containing epoxy compounds are capable of combining chemically with the resin/hardener system.
Flexibilisers. These are long chain molecules which may either cause a mechanical plasticising action (often referred to as plasticisers) or react to some extent with the resin during cure to increase flexibility by basically neutralising the attraction between adjacent chains. They are used to improve the impact resistance, peel strength or flexibility of epoxies but can cause side effects such as reduced tensile strength and transition temperature. They may be resinous in nature or be derived from curing agents such as polysulphides.
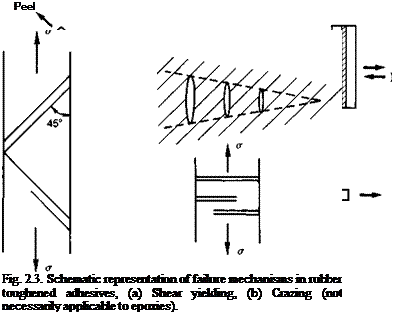
Tougheners. Unmodified epoxy systems tend to be strong in shear, compression and tension but brittle when cleavage or peel forces are imposed (Fig. 2.2). In general, adhesive joints are designed to avoid the latter forces but in practice they can rarely be eliminated entirely. Flexibilising can produce improvement in these properties at the expense of cohesive strength but in recent years a technique known as ‘toughening’ has been developed to overcome this problem. Toughening is achieved by the inclusion of a dispersed rubbery distortable phase within the load-bearing glassy matrix of the adhesive (Fig. 2.3). The aim is to provide a physically separate but chemically linked zone which absorbs fracture energy and prevents crack propagation. The analogy with drilling out the crack tips in metal is often drawn. The main energy absorption mechanisms are shear yielding in the matrix, crazing in the matrix and deformation
 |
(a) (b)
and failure in the rubber particles (Fig. 2.3). A common toughening agent is liquid carboxyl-terminated butadiene acrylonitrile (CTBN) rubber which maintains the stiffness, hardness and temperature resistance of the adhesive much better than liquid plasticisers or flexibilisers. Both two-part and single-part epoxies can be toughened
in this way(7), although it is more difficult to achieve a well dispersed rubbery phase in polymers cured at ambient temperature.
Adhesion promoters. Sometimes referred to as coupling agents, these additives have the ability to enhance resin adhesion to surfaces such as glass or metals(8). The most popular type are silanes which can either be mixed with the adhesive itself or applied to the substrate as a primer. They will be further considered in Chapter 3 under adhesion and surface pretreatment.
Commercially available resins may also contain fire retardants, anti-oxidants, surfactants, and so on. It may thus be deduced that useful adhesives are complex and sophisticated blends of many components. Not only the choice of hardener but also the presence of additives may affect significantly the physical and mechanical properties of both the freshly mixed and hardened adhesive. A model which is often used to represent the structure of thermosetting cross-linked adhesives is shown in Fig. 2.4(9). With epoxies the resin is represented by ‘hooks’ of high molecular weight and the hardener by the ‘eyes’ of short chain cross-linking segments) 10). An increase in hook or eye length or a decrease in the number of linkage points will result in an increase in elongation, peel strength, impact strength, low-temperature performance and permeability to water; it will also result in a decrease in elastic modulus, hardness, thermal stability, hot strength and resistance to chemical attack.
The rate of cure is temperature dependent and many formulations stop curing altogether below a temperature of about 5 °С. If carefully formulated the change in volume between the uncured resin — hardener system and the fully cured polymer can be very low. This property, together with their relatively high strength and claimed resistance to moisture and chemical attack, forms the basis of the use of epoxy resins as structural adhesives.
 3 июля, 2015
3 июля, 2015  Malyar
Malyar  Опубликовано в рубрике
Опубликовано в рубрике 