In Europe, in 2006, the large-scale recycling of paper, board and paperboard containers accounted for more than 58 millions tons of recycled paper products (Figure 8.44) [43].
Although adhesives enter the recycling process along with paper products, they must not disturb the process. The impact of adhesives on recycling operations
|
Figure 8.44 Paper recycling in Europe. |
 |
depends on the physico-chemical properties of the adhesive films themselves [44], because recovered paper contains adhesive in the form of a cured film (Figure 8.45). Usually, it is best to sort the adhesive films out as early in the process as possible.
An important property of adhesives with regard to recycling is their water solubility or redispersability, because papers are recycled in aqueous media. With few exceptions, hot melts that are basically derived from synthetic thermoplastics are totally insoluble in water. Waterborne adhesives may be either colloidal solutions (e. g. based on animal protein, starch, or polyvinyl alcohol) or dispersions. In the paper sector, dispersion adhesives are the most important group of adhesives used (on a quantity basis). The basic polymers of these synthetic thermoplastics are also insoluble in water; hence, in order to obtain a liquid phase and to enable them to wet a substrate, they must be ‘hydrophilized’ by means of emulsifiers or protective colloids, allowing them to be dispersed in water. During the setting of an adhesive the primary particles ‘flow’ into each other to form a solid film; the water-soluble protective sheaths are then incorporated into the film in such a way that dispersion-adhesive films are always somewhat hydrophilic. The composition of dispersion-adhesive films has an influence on solubility and redispersability that strongly depends on the pH value and the temperature of the water. This also holds true for colloidal adhesive solutions [45].
With regards to recycling, it is useful to distinguish between thermoplastic and nonthermoplastic adhesives. In the recycling of paper, the adhesive film is exposed to rather high temperatures (e. g. 40-50 °C in the pulper, 80-90 °C in the disperser, and 80-120 °C in the dryer). The majority of physically setting adhesives are thermoplastic. Thermoplasticity is defined as the ability of a material to melt to a liquid when the temperature rises. Whereas, low-molecular-weight substances melt at a defined temperature point, high-molecular-weight materials such as adhesives melt within a temperature range; moreover, as adhesives usually are a mixture of several different polymers this range may be quite large. Whether an adhesive is thermoplastic, or not, depends on its molecular structure. Usually, high-molecular-weight and cross — linked systems are not meltable and are hence nonthermoplastic (e. g. celluloses,
crosslinked polyurethanes, epoxy resins). Adhesives may also be nonthermoplastic if they begin to decompose at a temperature which is required for melting (e. g. animal protein, starch). The ‘softening’ behavior of a thermoplastic substance is due to the molecular mobility that increases with rising temperature, and which also improves its ability to build ‘adhesion bridges’. Soft, thermoplastic substances therefore feel sticky to the touch, and their molecular structure defines the temperature at which this effect occurs. In the case of high-molecular-weight thermoplastics (such as many technical plastics) this effect may occur at temperatures of more than 200 ° C, whereas other systems (e. g. polyacrylates) — and particularly mixtures — begin to show this effect below a temperature of 0 ° C. The Tg-value is a useful parameter for monitoring the ‘softness’ of a thermoplastic (the temperature range in which the polymer softens) as it describes the temperature at which a thermoplastic ‘melts’ internally, resulting in a high molecular mobility. High-molecular-weight thermoplastics are still relatively cohesive above their Tg, and still form mechanically stable films. Adhesive films that are sufficiently soft at room temperature to build up proper adhesion are known as ‘PSAs’ because they wet the surfaces to be bonded when pressure is applied. The ‘softness’ of the films ensures ‘flowing’ under pressure, resulting in proper adhesion. With increasing temperature, there will be loss in cohesion until eventually, even high-molecular-weight systems will melt. The abovedescribed phenomena apply to both hot-melt adhesives applied as thermoplastics, and to aqueous systems or solvent-based adhesives following setting. Paper recycling is basically a mechanical process, and therefore the thickness of the adhesive film greatly influences how easily it can be sorted out. Whereas sorting-out is no problem for thick films, thin films are often destroyed and their removal becomes more difficult.
However, thick, compact films generally do not comply with the paper manufacturer’s option. If only for cost reasons, the amount of adhesive used should be as low as possible, and for this reason computerized state-of-the-art nozzle systems that provide for high-precision, extremely economical and clean application of adhesives are widely used (Figure 8.46).
This not only saves adhesive and keeps the production process trouble-free, but these systems also have ecological advantages. Closed systems require substantially
|
Figure 8.46 The high-precision application of dots of a dispersion adhesive. |
less cleaning efforts and produce few waste products or wastewater. Yet, even if these adhesives are mechanically stable and water-insoluble, the films generated by the droplets can be too small to be sorted out in paper recycling facilities. Provided that these aspects are taken into consideration, adhesives can be chosen for the majority of applications in such a way that trouble-free paper recycling becomes possible.
 12 декабря, 2015
12 декабря, 2015  Pokraskin
Pokraskin 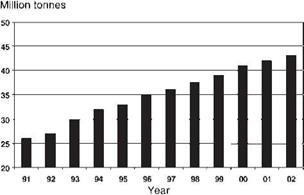

 Опубликовано в рубрике
Опубликовано в рубрике 