5.6.1
Chemistry of Polyurethanes
As the chemistry and reactivity of isocyanates are described in detail in several textbooks of organic chemistry, only those reactions relevant to polyurethane adhesives will be detailed in the following sections. The reactivity of isocyanates is based essentially on the electrophilic character of the carbon atom in the cumulated double-bond system ofthe isocyanate group. The resonance structures are illustrated in Figure 5.18.
R—N=C=0 •*—— ► R—N=C-Ol ■*—— ► R—N—C=0
Figure 5.18 Resonance structures of the isocyanate group.
Isocyanates react with other substances either by polyaddition or polycondensation. In the manufacture of polyurethane adhesives, the polyaddition reaction is used; the reactivity of the substances added depends on the nucleophilic character of their reactive group. The reactivity ofthe attacking group decreases in the following order: aliphatic amines > aromatic amines > alcohols > phenols > thiophenols. The steric hindrance of the molecules also has an influence on the reactivity of the nucleophilic reagents. For example, the reactivity decreases from primary to tertiary alcohols. The most popular method investigated and used commercially to date is the generation of polyurethanes by means of a reaction of diisocyanates or polyisocyanates with primary monoalcohols, dialcohols and polyalcohols (Figure 5.19).
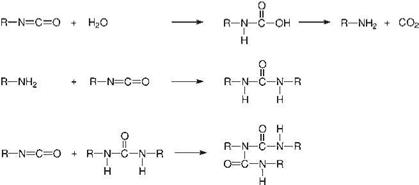
In the presence of moisture, another two-stage reaction takes place. In the first step, an unstable carbamic acid is formed, while in the second step carbamic acid
|
Figure 5.19 The reaction of isocyanate with alcohol. |
|
Figure 5.20 The reaction of isocyanate with water, and consecutive reactions. |
splits off carbon dioxide to give an amine (Figure 5.20). The amine then reacts with a further isocyanate group to give a urea group. At room temperature the urea group in turn reacts with free isocyanates, thereby crosslinking the polymer chains to form biurets, as it has a much higher reactivity than the urethane group [29-32].
5.6.2
 11 сентября, 2015
11 сентября, 2015  Pokraskin
Pokraskin 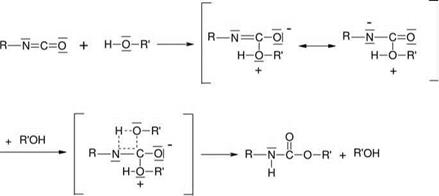
 Опубликовано в рубрике
Опубликовано в рубрике 