The above-described removable systems are based upon PSAs, the load capacity and creep resistance of which are limited (see Section 5.1). For the development of removable, high-strength adhesives, other solutions must be found. There are three main mechanisms that lend themselves to the removal of an adhesive bond.
First, severe subsequent embrittlement of the adhesive within its layer has been known to weaken the joint (or to cause overall delamination) since the 1960s, when small amounts of low-molecular-weight phenolic resin were added to solvent — containing neoprene-based contact adhesives to increase their long-term durability. Unfortunately, this well-intended increase of the phenolic resin content induced delamination of the bond (e. g. in doors) after about 10 years; this effect was explained by the continuous subsequent crosslinking of the then-dominant phenolic resins,
which increasingly embrittled the adhesive. Attempts to initiate subsequent embrittlement on demand — that is to say to ‘incorporate’ a subsequent crosslinking option into the adhesive — have failed to date [125], although the present investigations seem to be on the right path.
Second, adhesion promoters that are destroyed on demand would represent an optimum possibility also to remove a high-strength bond, but these have not yet been developed either.
The third intelligent possibility (besides thermal weakening or cold embrittlement) would be to mechanically destroy the bond-line on demand by using additives incorporated in the adhesive; these would expand greatly under external influences that would not occur in ordinary use, thereby breaking the adhesive joint. The first investigations with toluene sulfonic acid hydrazine, which splits off low-molecular — weight compounds at elevated temperatures, failed. However, when encapsulated foaming agents — which expand by physical change of the filler (e. g. evaporation at defined temperatures) — were used, the bond-lines created with dispersion adhesives (like those used to lay PVC floor coverings) were successfully weakened. The expansion was initiated by microwave irradiation; some test results are illustrated in Figure 8.101 [126].
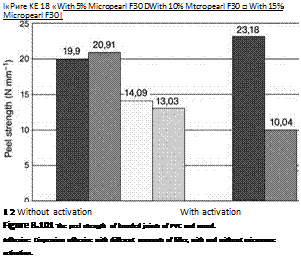 |
The mere addition of different amounts of Micropearl F-30 (an expanding filler) (Follmann) to dispersion adhesives reduced the peel strength of the bond due to fracture mechanics. If, in addition, the bond was exposed to microwave irradiation, there was mainly a drastic fall in peel strength, with the adhesive being found (in large part) on the surface of the wooden substrate, which was a desired effect. Storage of the filled adhesive for several months did not impair the foaming effect. However, the question remained as to whether this would still be the case ifthe adhesive bond-line were to be exposed for long periods of time to the conditions prevailing in actual applications of the building industry.
Attempts to ‘burst open’ crosslinked adhesives with the aid of these fillers have failed [127]. Whilst it has not yet been decided which of the above-mentioned (or other) methods should be investigated further, one thing is certain — removable adhesive bonds represent a significant step forward, even if they are rarely created by simple means.
 24 января, 2016
24 января, 2016  Pokraskin
Pokraskin  Опубликовано в рубрике
Опубликовано в рубрике 