Due to the reactivity of RFL systems, RFL-dipped textiles must be stored in a cool, dark and dry place (common storage conditions for such textiles) to preserve the adhesion-promoting effect until processing. Occasionally, correctly stored textiles preserve their properties for an extraordinarily long period of more than 24 months [60]. If, however, the textiles are exposed to UV light and sunlight, ozone and moisture, for example, the durability ofthe adhesion-promoting effect is rapidly and severely impaired (already after a few hours) because the RFL system is adversely
|
Figure 8.69 Possible reactions of precondensed resin with cellulosic structures in rayon. |
|
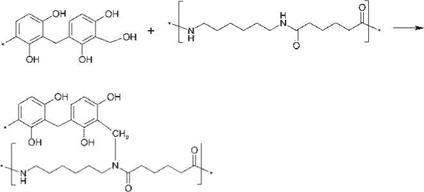
Figure 8.70 Possible reactions of precondensed resin with acid H-atoms in the amide group of PA 6.6. |
affected. Investigations performed by the author have shown that, following irradiation with daylight for 100 h, the adhesion-promoting effect decreased by 60%, while ‘normal’ storage without protective black foil for 100 h induced a decrease of 50%.
Based on results obtained with investigations performed with ozone and UV light in other laboratories [61], it can be deduced that the dramatic adverse effect was due to a degradation of double bonds on the surface of the dip film. During vulcanization with the rubber matrix, due to a lack of double bonds, curing was drastically reduced or made impossible. The failure pattern changed from ‘adhesive’ failure to cohesive failure.
Moisture itself does not have any significant influence on the degradation of the adhesion-promoting effect over time, but it does increase the adverse effect of ozone. If textiles are too moist, problems may arise due to bubble formation during vulcanization, which may impair the quality of the product, but not adhesion itself. Due to high moisture uptake (up to 13%), problems are most significant with rayon, followed by nylon with a moisture uptake of up to 6%. Half of the uptake occurs very rapidly (within 30 min after leaving the stove) and is completed after about 15 h.
In the case of excess storage, if tests reveal a decrease in adhesion due to oxidative degradation, it is possible to re-dip the textiles to avoid economic loss. The original adhesion-promoting effect can be restored because there is still a sufficient amount of reactive components in the resin.
Textile finishing for the manufacture of fabric-reinforced rubber goods has been an established process for many decades, and indeed all technical fields of application of high-performance fibers rely upon this process. However, the phenomena of adhesion that underlie this process have not yet been fully identified, most likely due to the complexity of the reaction components used. Details of standard formulations and RFL systems, as well as the corresponding rubber mixtures, are publicly available, although the formulations of mixtures actually used in practice are kept as ‘trade secrets’ because they play a decisive role in the high performance of rubber-textile composites, and hence in the economic success of the involved businesses. Continuous development aiming at solving existing problems will doubtless meet and
exceed the growing need for technical performance of reinforced rubber systems. With environmental protection in mind, one of the priorities today is to eliminate potentially harmful chemicals contained in the formulations, such as formaldehyde.
 9 января, 2016
9 января, 2016  Pokraskin
Pokraskin 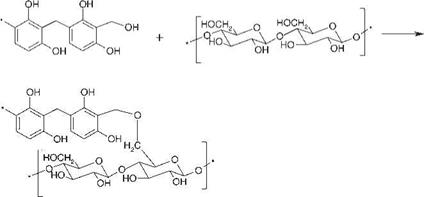
 Опубликовано в рубрике
Опубликовано в рубрике 