The existence ofadhesion promoters has long been known. For example, as far back as the eighteenth century, Henry Duhamel du Monceau (see Chapter 2) explained that the adherence of glue to wood could be improved by rubbing the wood with garlic before the glue was applied. Whilst this was mere speculative experience, reactive organosilanes [42, 46] have become standard adhesive promoters on the basis of a detailed knowledge and description of their chemistry and mode of action. In the following, the relevant information will be confined to adhesive bonding technology.
Organosilanes are bifunctional silicon compounds wherein the Si atom is substituted three organic groups that can be transformed into OH-groups by hydrolysis, as well as an organic, mostly chain-like molecular fragment that, at the end facing away from the Si atom, carries a reactive group capable of forming bonds with adhesive molecules. The reaction scheme and the adhesive mechanism are illustrated in Figure 5.37.
As shown in Figure 5.37, the hydroxyl groups required for the addition reaction with the substrate are also capable of a condensation reaction with another silane molecule to yield a silicone. This reaction starts without any catalyst, and must be prevented before using these compounds as adhesion promoters. Therefore, the silane is blocked with the organic groups (-OR) shown at the top of the reaction scheme, and it becomes storage-stable. Water is added only shortly before application, and hydrolytic activation takes place. Catalysts may accelerate this process. The proneness of the promoter to polycondensation at application, or in the applied layer,
Organosilane
x Y-(CH2)„-Si(OR)3
j + H20 (+ catalyst)
1 — ROH
+
Si lanol
x Y-(CH2)n-Si(OH)3
I
I — Z H2°
Polysiloxane
Y Y Y Y Y Y
I I I I I I
(CH2)n (CH2)n (CH2)n (CH2)n (CH2)n (CH2)n
I I I I I I
—Si—О—Si —О—Si—О—Si—О—Si—О—Si —
I I I I I I
0 0 0 0 0 о
1 I I I I I
HO—Si-O-Si————— Si—О-Si — o—Si -0-Si —
HO I I I I I
ОН О OHOH о но о HO
‘_______________________ I I____________________ I_________________________________________ I I
-M—O-M-O-M-O-M-O-M-O-M-O-M Metal surface
Figure5.37 Hydrolysis and condensation of an alkoxysilane at the surface of an inorganic substrate.
cannot be prevented completely, so that a silicone-like intermediate layer is formed within the assembly that may impair its load capacity if too large a quantity is applied.
In silane adhesion promoters, the spacer groups between the reactive groups can be of a different nature. Indeed, their chemical structure and length have been shown to be primordial for the reactivity and functionality of the silane.
These adhesion promoters are mainly used for the bonding of glass as they provide high durability. However, when applied to the surfaces ofother inorganic substances, they often also enhance the long-term durability of the bond, particularly on metal surfaces where hydroxyl groups provide for chemical reactions [47]. However, silane adhesion promoters are not capable of protecting the bond against bondline corrosion, which is a potential risk factor in the case of metal bondings (see Section 7.5).
There has, therefore, been good cause for giving some thought to other types of adhesion promoter, without foregoing the principle of specific bifunctionality. An important approach here is to replace the silanol configuration by molecular groups capable of forming chelate compounds with metal surfaces, or rather with their oxides and hydroxides. These chelate compounds may have a high resistance to water; an example is shown in Figure 5.38.
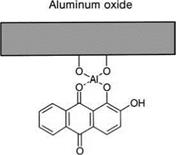 |
Figure 5.38 Chelate bond between alizarine (dihydroxyanthraquinone) and aluminum oxide.
As early as the 1970s, it was shown that simple chelate-complexing agents such as alizarin (dihydroxyanthraquinone) (Figure 5.38) or morin, once adsorbed onto aluminum surfaces from alcoholic dilutions, promote adhesion in phenolic resin adhesive bonds [48]. At that time, it was supposed that the adhesive, with its reactive methylol groups, was added to the free OH-groups of the chelate complexing agents. It was also shown that the aluminum oxides have an increased resistance to hydration when chelate complexing agents are used.
Later, it became possible to add spacers and, for example in the case of epoxy resins, to add compatible epoxy or amine groups to those substances [49, 50], generating adhesion promoters (Figure 5.39, left) that considerably improved the age resistance of aluminum bonded with these adhesives.
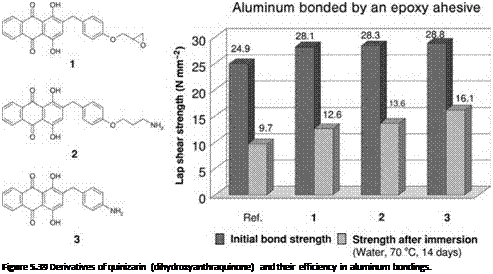
As noted in Chapter 3 (where polyhydroxy-benzoic acid was referred to as a standard example), chelate-complexing agents may also be added to other metals, for
|
Figure 5.40 Derivatives of polyhydroxy-benzoic acid and their efficiency in steel bondings [50]. |
example iron alloys, with high resistance to water. Also, when bonding steel surfaces with amine-curing epoxy resin adhesives, adhesion is significantly enhanced if spacers and groups that are reactive with regard to epoxy resin adhesives are added to the chelate-complexing agents of the adhesion promoter (Figure 5.40).
Chelate-complexing agents are a good example of the immense development potential inherent to adhesion promoters. Adhesion promoters represent a matter of special importance because they can replace costly surface preparation methods.
 30 сентября, 2015
30 сентября, 2015  Pokraskin
Pokraskin 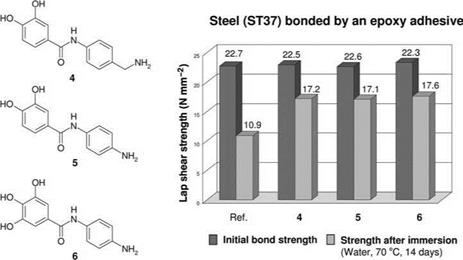
 Опубликовано в рубрике
Опубликовано в рубрике 