Distillation of perfume ingredients from their natural sources can be done in three ways: dry (or empyreumatic) distillation, steam distillation or hydrodiffusion. Dry distillation involves high temperatures, since heat (and in most cases this is direct flame) is applied to the surface of the vessel containing the plant material. Usually this technique is reserved for the oils of highest boiling point, typically those derived from wood, because the high temperatures are necessary to vaporize their chemical components. Cade and birch tar are the major oils obtained by dry distillation. Cade and birch tar oils contain distinctive, burnt, smoky notes as a result of pyrolysis of plant material. In steam distillation, water or steam is added to the still pot and the oils are codistilled with the steam. The oil is separated from the water by means of a Florentine flask, which separates them based on their differing densities. Figure 3.1 shows a simple schematic representation of a still and a Florentine and Figure 3.2 shows a still charged with jasmine flowers ready for the top to be fitted prior to distillation.
The waters that со-distil with the oil are called the waters of cohobation. In most cases, these are a waste product and are either
|
Hook for lifting lid when refilling
Figure 3.1 Still and Florentine flask |
|
Figure 3.2 Still charged with jasmine flowers |
discarded or recycled to the still pot. The waters of cohobation obtained from rose distillation are different. Rose oil is somewhat water soluble and so the ‘rose water’ is kept as a perfume and flavour ingredient. The presence of water in the pot during steam distillation limits the temperature of the process to 100 °С. This means that much less degradation occurs in this process than in dry distillation. However, some degradation does occur. For example, tertiary alcohols present in the plant often dehydrate in the pot and distil as the corresponding hydrocarbons.
Hydrodiffusion is a relatively new technique, and is essentially a form
of steam distillation. However, it is steam distillation carried out upside down since the steam is introduced at the top of the pot and the water and oil taken off as liquids at the bottom.
Perfume materials obtained in this way are referred to as essential oils. Thus, for example, the oil obtained by steam distillation of lavender is known as the essential oil of lavender, or lavender oil. Sometimes, the monoterpene hydrocarbons are removed from the oils by distillation or solvent extraction to give a finer odour in the product. The process is known as deterpenation and the product is referred to as a terpeneless oil. This is, of course, a misnomer since, for example, the major component of lavender oil terpeneless is linalyl acetate, a monoterpene.
 27 июня, 2015
27 июня, 2015  Malyar
Malyar 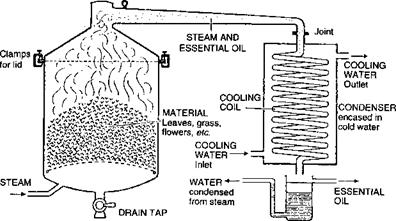

 Опубликовано в рубрике
Опубликовано в рубрике 