So, plants and animals produce odorous materials for a wide variety of reasons, but how do they generate them? All living organisms produce chemicals through a process known as biosynthesis. The materials thus produced can be classified into two major groups, viz. primary and secondary metabolites. Primary metabolites are those that are common to all species and can be subdivided into proteins, carbohydrates, lipids and nucleic acids. The materials used as perfume ingredients are mostly secondary metabolites, though a few are derived from primary metabolites by degradative processes. The four categories of secondary metabolites, in decreasing order of importance as sources of perfume ingredients, are terpenoids, shikimic acid derivatives, polyketides and alkaloids. Very few odorous materials are derived from the alkaloid family, so these are not discussed further here. Of the others, the terpenes are, by far, the most important. The terpenoids, shikimates and polyketides are all originally derived from glucose (Scheme 3.1; in this scheme and subsequent ones, the letter P is used to represent a single phosphate unit). It is worthwhile spending some time considering how the natural perfume ingredients are put together since, through this, the patterns of nature can be understood and used to assist in identifying the structures of newly isolated materials and in producing new compounds with similar odour properties. More detail on biogenesis is given in the books by Bu’Lock and Mann et al. (1994).
![]()
![]()
![]()
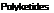
![]()
![]()
Scheme 3.1
Green plants and photosynthetic algae synthesize glucose from carbon dioxide and water using sunlight as the energy source to drive this energetically unfavourable process, which is known as photosynthesis. Glucose can be broken down, either by the plant which made it or by another species which obtains it by eating the plant, to give the enol form of pyruvic acid, in which the enolic hydroxyl group is protected by formation of a phosphate ester. One metabolic pathway builds shikimic acid from the phosphoenol pyruvate and another converts it into acetyl coenzyme-A. The thiol function of coenzyme-A serves both as an activating group and as an efficient leaving group, thus making aldol — type chemistry facile and leading to long-chain compounds in which every second carbon existed, at some point, as a ketone. Self-condensation of these chains leads to the polyketides. Acetyl coenzyme-A can also be used to synthesize mevalonic acid, precursor to the terpenoids.
Lipids and polyketides are biosynthesized by aldol-type reactions of esters with coenzyme-A, as shown in Scheme 3.2. The coenzyme-A ester of a fatty acid undergoes reaction with acetyl coenzyme-A to give
a /Mcetoester. Reduction of the ketone group followed by elimination of the resultant alcohol and addition of hydrogen gives an acid with two carbon atoms more in the chain. This is why natural fatty acids contain even numbers of carbon atoms in their chains. If the poly — ketoacids undergo condensation reactions rather than reduction, the result is a phenolic material of the polyketide family, as in the formation of orsellinic acid (Scheme 3.3), which is the precursor for some odorous components of plants.
о
Оч IIНО. /
![]() у со2н он
у со2н он
Orsellinic acid
Scheme 3.3
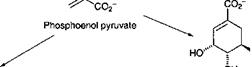
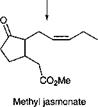
One lipid of interest is arachidonic acid. This polyunsaturated fatty acid undergoes a radical cyclization reaction involving oxygen, as shown in Scheme 3.4. This cyclization leads to an important group of compounds known as prostaglandins, hormones in the animal kingdom. Degradative reactions lead to shortening of the chains to give jasmonic acid, a plant hormone and precursor for two important odorous materials, jasmone and methyl jasmonate.
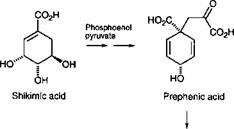
Addition of phosphoenol pyruvate to erythrose-4-phosphate leads, through a number of reaction steps, to shikimic acid. The 3,4,5- trihydroxybenzoic skeleton of shikimic acid occurs in many perfume components, although the oxygen atoms in the product are not usually those of the original shikimic acid. The original oxygen atoms are lost during biosynthesis and others reintroduced into the same sites at a later stage by oxidation. Addition of a further unit of phosphoenol pyruvate adds a three-carbon chain to the carbon carrying the carboxyl group. The latter is then lost by decarboxylation. An abridged scheme for the biosynthesis of eugenol, the characteristic odorant of cloves, from shikimic acid is shown in Scheme 3.5.
Terpenes are defined as materials made up of isoprene (2-methyl- butadiene) units. In the perfume industry the word ‘terpene’ is often used incorrectly to refer to monoterpene hydrocarbons. However, the term does include all compounds derived from the connection of two isoprene units to give a 10-carbon skeleton. The names given to the other members of the terpene family are shown in Table 3.1
|
‘OH Jasmonic acid |
|
|
|
P0^C02H
Phosphoenol
pyruvate
HO
 PO OH Erythrose-4-phosphate
PO OH Erythrose-4-phosphate
|
|||||||||
|
|||||||||
|
Name |
Number of isoprene units |
Number of carbon atoms |
|
Hemiterpenes |
1 |
5 |
|
Monoterpenes |
2 |
10 |
|
Sesquiterpenes |
3 |
15 |
|
Diterpenes |
4 |
20 |
|
Sesterterpenes |
5 |
25 |
|
Tri terpenes |
6 |
30 |
|
Carotenes |
8 |
40 |
|
Steroids |
Terpenoids which produce Diels’s hydrocarbon when distilled from zinc dust |
|
Table 3.1 Classification of ter penes |
|
OH |
![]() ґЬр
ґЬр
|
|
||
|
|
||
OPP
Pentenyl pyrophosphate
Scheme 3.6
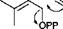
Scheme 3.6 illustrates how, through phosphorylation, elimination and decarboxylation, mevalonic acid is converted into isopentenyl pyrophosphate, which can be isomerized enzymically into pentenyl pyrophosphate. Coupling of these two isomeric materials gives geranyl pyrophosphate, as shown in Scheme 3.7. Addition of a further molecule of isopentenyl pyrophosphate gives farnesyl pyrophosphate. These coupled units then lead to the monoterpenes and sesquiterpenes, respectively. Addition of further units of isopentenyl pyrophosphate leads in the same manner, to the higher terpenes. The reactions shown in Scheme 3.7 give rise to what is referred to as the head-to-tail coupling, in which the ‘head’ of one isoprene unit is connected to the ‘tail’ of another. This is, by far, the most common way of joining isoprene units together, though tail-to-tail couplings also occur, the best example being the tail-to-tail fusion of two geranylgeranyl pyrophosphate units to produce squalene and the carotenes. The terpene pyrophosphates undergo cyclization reactions under the influence of
appropriate enzymes. Other enzymes then carry out further chemical conversions, such as oxidation, on the terpenes. This leads to a vast array of complex structures, the final structure depending on the exact nature of the enzymic reactions involved. Since the enzymes are often unique to one species, the terpenes (and of course, other metabolites also) produced by a plant can be used by botanists to classify it. Such classification of plants is referred to as chemotaxonomy.
 OPP
OPP
Pentenyl pyrophosphate Isopentenyl pyrophosphate
I ——————- —► Monoterpenes
1 1 OPP
Geranyl pyrophosphate
![]()
OPP
etc.
Scheme 3.7
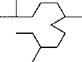
Scheme 3.8 shows how the isoprene units and the original backbone can be traced out in a number of terpenes that are important in perfumery. Sometimes skeletal rearrangements occur which make this process more difficult and fragmentation or degradation reactions can reduce the number of carbon atoms so that the empirical formula does not contain a simple multiple of five carbons. Nonetheless, the natural product chemist quickly recognizes the characteristic terpene framework of the structure.
head tail head tail
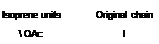
 Terpene (name and source) Structure
Terpene (name and source) Structure
OAc
![]() Linalyl acelate Lavender oil
Linalyl acelate Lavender oil
a-Pinene
 |
|
Turpentine
The book by Mann et al. (1994) on natural products provides a good introduction to the biogenesis of natural perfume ingredients and the review by Croteau (1987) gives further detail on the biosynthesis of monoterpenes.
 26 июня, 2015
26 июня, 2015  Malyar
Malyar  Опубликовано в рубрике
Опубликовано в рубрике 