Consider now an experiment which demonstrates molecular adhesion simply and unequivocally. Take a sample of very fine, pure solid powder, say 4 g of 0.2 pm diameter grains of titanium dioxide. This powder can be poured into a hard steel pelleting die, as shown in Fig. 2.9(a). Coulombic forces can be prevented by the presence of a radioactive source to ionise the air and leak away any stray electrons.
After pressing the powder with a force, say 100 kN, the pellet can be ejected from the die in one piece. The powder particles then stick together with considerable strength. Of course, the tablet is porous because the titanium dioxide grains are so hard that they cannot squash together plastically. But where the powder particles touch each other, the molecules of titanium oxide are in close proximity as a result of the large force which has urged them into molecular contact. Then, the short-range adhesion forces discussed above can act to pull the particles strongly together, thereby resisting external stresses.
By testing the pellet in bending or tension, it is easy to find that the compacted pellet has the properties of a porous titania ceramic. It is strong, elastic, and brittle, but not so strong as dense transparent titania which has no pores to weaken it. Typically, the pellet contains 50% by volume of air cavities,
|
Fig. 2.9. (a) Loose powder loaded into steel die; (b) powder compressed by die; (c) pellet after release from die. |
which make the pellet crack readily. Further evidence of molecular adhesion is obtained by heating the pellet in a furnace. At temperatures around 1200°C, the particles begin to diffuse, and the molecular adhesion within the pellet is so powerful that the pellet gradually shrinks to exclude the pores and become dense. The molecular adhesion forces are sufficient to pull the grains into complete and perfect contact. This process is the sintering phenomenon.
William Hyde Wollaston15 first described this type of adhesion experiment in 1829. He was interested in making dense and strong wires from platinum and other rare metals, such as palladium and osmium, which he had just discovered. Platinum is so hard and refractory that it is extremely difficult to work by ordinary melting and casting techniques. Wollaston prepared the platinum in fine particle form by precipitating the metal from an acid solution which had been used to remove impurities. This produced a muddy mixture of water and particles which were cleaned by washing, then dispersed by milling in a wooden mortar and pestle. Wood was used to limit contamination since it would burn out later.
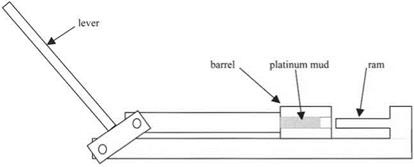
The difficulty was converting the fine metal powder into a dense block. To achieve this, Wollaston needed to get the particles sticking together in a dense packing. First, he constructed a mechanical press, shown in Fig. 2.10, consisting of a brass cylinder into which fitted an iron piston around 25 mm diameter. The barrel of the cylinder was tapered so that the pellet could be ejected after compaction. After placing the platinum mud in the barrel, then covering it with blotting paper to allow the water to soak out, and greasing the piston with lard, Wollaston pressed on the ram to apply a force of about 30 tonnes weight to the powder mass. This force was sufficient to increase the packing of the fine particles under the ram from a loose state of 20% packing to a porous pellet near 50% dense. This pellet was ejected in one adherent piece and was “hard and firm” suggesting that each platinum particle was adhering to its neighbors by molecular forces.
|
Fig. 2.10. Wollaston’s press for compacting platinum powder together into an adhering mass. |
Heating of the pellet with burning charcoal was sufficient to remove moisture and organic lubricant. Then the pellet was raised to white heat in a Staffordshire coke furnace. This caused the pellet to contract as the particles sintered together. Pounding the hot pellet with a hammer produced a material which was 99% dense and which produced platinum wire of the “highest tenacity.”
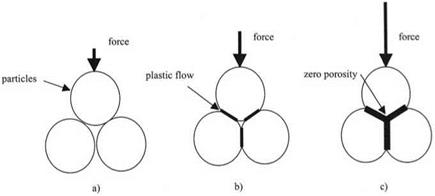
Of course, platinum is not as hard as titanium oxide and so the deformation of the particles during the compaction is more plastic. The adhesion between plastic particles during compaction is even more striking than that between elastic oxide grains. This is readily demonstrated by compressing potassium bromide powder in a steel die, as routinely done for infrared analysis. During the compression of the grains, the pressure at the contact points becomes larger than the yield pressure and consequently the contact spots enlarge until all the porosity has been excluded, as indicated schematically in Fig. 2.11.
On removal from the die, the compacted pellet is seen to be fully transparent and completely dense. The pellet is also very strong, elastic, and brittle, comparable to a piece of solid potassium bromide made by other methods. Thus the conclusion is that the compaction force has brought the grains into molecular contact, generating adhesion. Further force has sheared the material close to the contacts, allowing more intimate molecular contact until all the particle surfaces are adhering strongly. The lessons we learn from such demonstrations are fourfold: all bodies can be made to adhere together; finer particles stick more easily; force is usually required to push the bodies into molecular contact; and deformation, especially plastic or diffusive flow, allows more extensive contact to give maximal adhesion.
|
Fig. 2.11. Three stages in compaction of plastic particles: (a) low load, (b) higher load causes plastic deformation, (c) higher load removes all pores. |
Skeptics may say that the adhesion developed in the above experiment can be explained by other well-known ideas. These sorts of argument leveled against molecular adhesion phenomena can be listed. There is first the suction argument, which says that the particles are acting as rubber suction pads, and merely sealing around the edges; this is easily shown to be false because the pellets are just as strong in vacuum, where suction pads fall apart. Secondly, there is the mechanical keying argument, which suggests that the particles behave like Velcro, with little hooks and eyes to cause adhesion. This is easily answered because when you look at the surfaces by electron microscopy, the particles are often extremely smooth and shiny. There are no hooks and eyes. Moreover, the smoother particles show stronger adhesion than rough particles. A third argument is that the particles are electrically charged, to give electrostatic attractions. This is readily disproved by doing the experiment in the presence of ionizing radiation to leak away the electrons. The adhesion is not affected. The fourth argument is moisture; a critic will say that the particle surfaces are damp and this is like the adhesion of wet sand, which sticks more than dry sand. Again this can be disproved by evacuation; in fact, the dryer the particles, the better they adhere. Finally, a desperate argument is Newton’s gravitation. But a rapid calculation shows that this is much too small to cause the adhesion observed. So the conclusion must be that molecular adhesion is the most rational explanation of the experiments described above. There is obviously a force of molecular adhesion which draws bodies together; as a consequence there must therefore be an energy of adhesion, because energy is the summation of force acting over a distance. The experiments above define the force; to understand the energy we must now look at the range of action of the adhesion.
 1 сентября, 2015
1 сентября, 2015  Pokraskin
Pokraskin 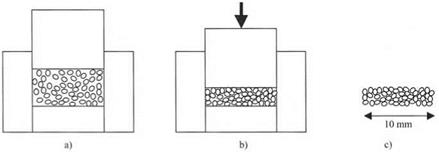
 Опубликовано в рубрике
Опубликовано в рубрике 