Yet another confusing adhesive influence is that of electricity and magnetism. Adhesion of bodies as a result of electrical charging was known to the Greeks. Rubbing glass or amber with cloth would make the material attract small
pieces of dust or paper. A particularly eerie sensation is when your hair stands up as a result of electrical fields. The same effect is obtained by rubbing a balloon. The rubber material picks up an electrical charge, allowing the balloon to stick to a window. It is evident that such adhesion is different from true molecular adhesion because moisture, or nuclear radiation, allows the charge to leak away and the balloon drops off.
Priestley7 described such phenomena in 1767 in his book History and Present State of Electricity and suggested that the law of electrical attraction should be the same as Newton’s gravitational principle. This allowed Cavendish to provide an experimental proof four years later, but he did not publish his work and it was not found again for more than a century.8 Meanwhile, Coulomb in 1785 had constructed a mechanical balance for measuring the repulsive forces between two equally charged pith balls, showing that Newton’s law of inverse squares was indeed operating. The electrical force seemed to act from the center of the ball, and decayed with the square of the distance from the center. Thus such forces act over considerable distances and are easily distinguished from molecular adhesion forces, which act only a few nanometers from a surface.
Coulomb then showed by means of his mechanical balance that the inverse square law operated also for magnets. It later became clear that magnetism resulted from flowing electrical charges and that the inverse square law applied also to electrical currents, i. e. electrons flowing in wires. Thus, the field of electromagnetism became systematically understood in terms of Coulombic interactions, caused by very small charged particles, i. e. electrons and their flow. Electrons were shown to be ubiquitous in all known atoms and also to be responsible for chemical reactions.9 For example, the intense and fiery reaction of sodium with chlorine was shown to be the transfer of a single electron from one sodium atom to its neighboring chlorine atom, as shown schematically in Fig. 2.6. The main problem with electrons is that they are much smaller than atoms, so they cannot be treated by our ordinary concepts of particles. Instead, they have properties of both waves and particles and so must be described by quantum theory.9 The other problem is that electrons are negatively charged and therefore
|
Fig. 2.6. Schematic picture of reaction between sodium and chlorine atoms as an electron transfer. |
|
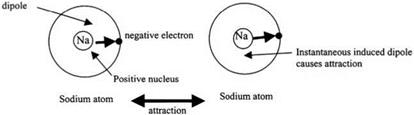
Fig. 2.7. London attractive force is caused by instantaneous dipoles induced in all atoms. |
repel each other. If electrons repel, and atoms are bunches of electrons, how then can atoms be attracted towards each other?
The answer to this question is complex and is rooted in the quantum theory proposed by London10 in 1937, and summarized in several books on intermolecular forces.11,12 The principle can be understood by thinking of the outer electron in the sodium atom rotating rapidly around its nucleus, as shown in Fig. 2.7. Because the electron is negative and the nucleus is positive, at any instant there is a separation of negative and positive charge, even though the average charge separation is zero over a period of time. This is an instantaneous dipole, like a tiny magnet, which can line up a similar sodium atom at a distance. This gives an instantaneous dipole-dipole interaction which is always attractive. Such a “London” force is the cause of the general attractive adhesion between all atoms. London showed that this force was very different from simple electrostatic interactions because it dropped off with distance more rapidly, with separation to the power of 7. This explains why molecular forces are short range, and only operate when bodies are in close contact.
The conclusion from this brief discussion is that molecular adhesion is caused by electromagnetic forces, but not the simple forces that operate in electric motors or between magnets. Those Coulombic forces can be either attractions or repulsions and obey Newton’s inverse square law. By contrast, molecular adhesion is caused by London forces which are always attractive and which fall off extremely rapidly with separation.
In other words, molecules are made up of both electrons and protons which balance their charges exactly to give zero Coulombic adhesion. But the slight separation of charges in the molecules gives overall attractive London adhesion. The sub-atomic particles themselves adhere in quite a different way as described below.
 28 августа, 2015
28 августа, 2015  Pokraskin
Pokraskin 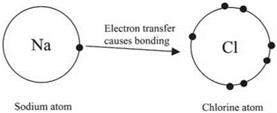
 Опубликовано в рубрике
Опубликовано в рубрике 