Although theories of adhesion have only been developed over this past century, the technological arts of adhesion processes have existed since prehistoric times. Man’s inventiveness in finding ways of sticking objects together to fashion beautiful structures is perhaps the finest illustration of human intelligence.
There is a wide range of products and processes, shown in Table 1.1, which utilize adhesion. Most of these processes and products have grown empirically from primitive beginnings which are now almost forgotten.
Perhaps the earliest and most striking example is the cave paintings discovered at Altamira in Spain and Lascaux in France in the nineteenth century.10 These paintings of bison and horses date from the last ice age, some 12,000-17,000 years ago, and illustrate the strong adhesion of fine pigments to the cave wall (see Fig. 1.9). Presumably, the ancient artists had ground up the
|
Table 1.1 Products and processes related to adhesion
|
colored minerals and plants to make a dispersion of fine particles in water to produce a paint which adhered successfully to the surface.
Another powerful invention was that of Indian Ink, a suspension of carbon black which, after drying became waterproof. Such inks came originally from China some 5000 years ago.11 Lampblack was prepared by burning pine wood and collecting the soot in a furnace container. The fine black powder from the top of the furnace was mixed with glue made by boiling animal skins, together with other additives like crushed pearl, egg white and musk, pounded 30,000 times to break up aggregates, then strained through cloth to produce a fine ink. This was a superb example of the interaction of the collagen polymer solution with the carbon particle surface, to give entirely new properties.
Of similar vintage was the discovery of the sintering process to make clay bricks which were weather-resistant. Mud and clay have been used as building materials for millenia. Clay in particular was important because of its mould-
|
Figure 1.9. Cave painting from Altamira, 12 000-17000 years old |
![]() ability when wet; it could be plastically formed into elegant and large shapes. After drying, the clay was very strong but suffered from the disadvantage that it became weak again on rewetting. This problem was cured by heating the clay articles to high temperatures such that the particles adhered more strongly together. Temperatures of 700° C were sufficient to prevent rapid degradation by water. But above 1100°C, the material became totally resistant to moisture.11 Such technology has been extended over the past century to produce ceramic materials of all kinds, from electronic packaging, to magnets for electric motors, to nuclear fuel pellets.
ability when wet; it could be plastically formed into elegant and large shapes. After drying, the clay was very strong but suffered from the disadvantage that it became weak again on rewetting. This problem was cured by heating the clay articles to high temperatures such that the particles adhered more strongly together. Temperatures of 700° C were sufficient to prevent rapid degradation by water. But above 1100°C, the material became totally resistant to moisture.11 Such technology has been extended over the past century to produce ceramic materials of all kinds, from electronic packaging, to magnets for electric motors, to nuclear fuel pellets.
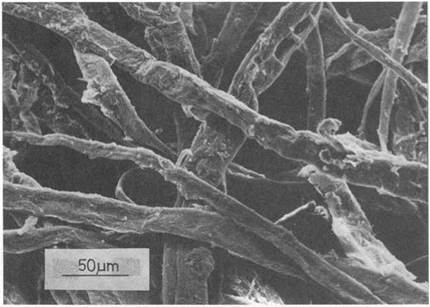
A somewhat later technology, the manufacture of paper, was created about 2000 years ago by the Egyptians, who found that beating papyrus reed stems together, then drying them, gave a matted fibrous surface which was ideal for writing.12 The cellulose fibers in the reed stems made good contact which hardened and strengthened as water was removed (see Fig. 1.10). Again, the problem was to waterproof the paper, and this can now be done by using synthetic polymer to stick the fibers more permanently. Later, mineral fibers like asbestos were used, but these proved to be a health risk when ingested. Now, there is a huge fibers industry, making polymer, glass and carbon textiles which can be adhered to give beautiful structures, such as fishing rods, cars, yachts, and the Millenium Dome.
Other ancient examples of the use of natural earths and minerals include the application of bitumen as a hot melt adhesive to stick rocks together, known in
|
Figure 1.10. Tracing of cellulose fibers ahering together in paper. |
biblical times, and that of volcanic ash as a cement which would harden and remain strong in the presence of water. The Romans wished to build durable seawalls and found that a certain ash from the region of Pozzuoli, so-called pozzolanic material, would harden after mixing with water and would not be weakened by further immersion in sea water. Some of the sea defences built with this material are surviving to this day. Interestingly, the pozzolanic cement has lasted better than the rocks in the original walls. It was not until 1824 that Joseph Aspdin managed to make such a cement synthetically by heating clay and limestone together in a furnace, then grinding the product to a fine powder which he called Portland cement. This has now been developed to be the largest synthetic material on the planet.13
A more recent adhesive technology which has only come to fruition in the 20th century is that of synthetic polymer latex. When Columbus travelled for the first time to the New World five hundred years ago, he found that the natives played games with a rubber ball, which they had made by gathering the natural latex from certain trees. This milky fluid exuding from the tree-bark could be dried and used in several interesting ways: as a glue to stick things together; as a waterproof coating for fabrics; or as an elastic material for ball games. The natural latex was somewhat unstable and putrescible, but once the trick of adding ammonia as a stabiliser was discovered, the latex could be stored, transported, and used for many applications.
The secret of artificially making such milky rubber dispersions was found by Hofmann14 and co-workers at Bayer in 1913. There was a need at that time to find substitutes for natural rubber for the manufacture of tires. By taking a synthetic rubber precursor, for example butadiene, which is an organic liquid, adding it to water in the presence of a dispersing agent such as blood serum, then shaking, a milky fluid like the natural polymer latex could be produced, as shown in Fig. 1.11. This has been enormously successful for producing the polymer materials which lie at the heart of our modern civilization. Typical applications are adhesives, paints, condoms, tires, window frames, clothes and shoes. Essentially, we have found a synthetic replacement for the natural sticky latex adhesive material we see in nature.
 21 августа, 2015
21 августа, 2015  Pokraskin
Pokraskin 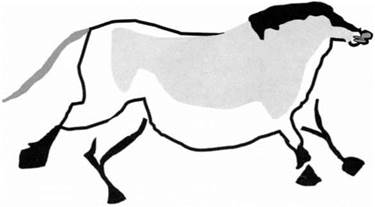
 Опубликовано в рубрике
Опубликовано в рубрике 