Consider the adhesion of two bodies on the Earth’s surface, as shown in Fig. 1.5. However much we clean or polish the surfaces to make them smooth and free from contamination, we realize that the contact between the bodies cannot be made easily at the molecular level. Magnifying a small portion of the contact region, by a factor of one hundred million, to observe the atomic structure of the material, it is evident that the atoms of the material do not make contact at all. In the first place, the surfaces are covered with layers of foreign atoms like oxygen and also by contaminating molecules such as water, which prevent true atomic contact between the solid bodies. Secondly, the surfaces are not smooth at the atomic scale, but are rough and jagged, like mountain ranges placed on top of
|
Figure 1.5. Two bodies in contact, with a small part of the apparent contact magnified to show the effects of contaminating atoms and of surface roughness. |
each other,6 so that large gaps exist to reduce the surface interaction down to an even lower value.
Thus, the picture of adhesion developed in Fig. 1.5 very much resembles the ideas generated by Newton about the laws of motion, (remember Fig. 1.3). The first law of adhesion, that all atoms adhere, is true in space when solid surfaces are smooth at the 0.1 nm dimension. However, in the Earth’s environment, we have to account for contaminant molecules, which reduce adhesion considerably. This is the second law of adhesion, described in more detail in Chapter 3. Both these effects are conservative, in that no energy need be lost. But there is another problem, that of surface roughness which reduces true contact enormously and which therefore diminishes adhesion even more. Roughness is an example of a mechanism, rather like viscosity in Newton’s theory, which is complex, variable, and capable of energy losses. Thus it is cannot be explained easily and must be debated at length. In addition to roughness, many other lossy mechanisms such as hysteresis, restructuring, etc., can be found, leading to a rich variety of adhesion phenomena. These are described in Chapter 7 onwards. Grouping all these mechanisms together, we conclude that there is a third law of adhesion: “the mechanism has a large influence on the macroscopic adhesion, even though the molecular adhesion remains constant.”
Summing up these arguments, it is evident that molecular adhesion has several layers of complexity leading to the adhesion paradox. Our macroscopic world of ordinary experience is seen to be false at the molecular level. All atoms and molecules adhere. The moral we draw from this is clear: we must distrust all artificial ideas which are glibly produced to explain adhesion. Ideas such as keying, adhesives, suction, and so forth are utterly unnecessary. Atoms stick together naturally. It is the most fundamental property of atoms to adhere. The odd and unexpected conclusion is that our ordinary experience of adhesion is close to zero, even though we theoretically expect the adhesion of atoms to be large. This brings us to ask the question, “How large should adhesion be in theory?”
 20 августа, 2015
20 августа, 2015  Pokraskin
Pokraskin 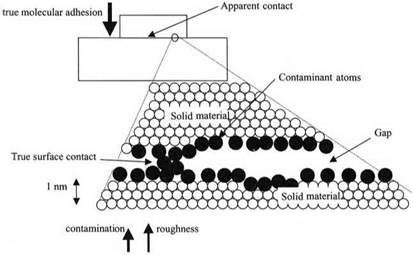
 Опубликовано в рубрике
Опубликовано в рубрике 