This book is about a paradox, the adhesion paradox, which is just as challenging as Newton’s ideas about motion and gravity. In fact, the adhesion paradox can be visualized in a similar way. Common experience tells us that ordinary objects do not stick together easily. A cup does not stick to its saucer; your foot does not adhere to the floor. Cathedrals were designed on the basis that the stones do not stick together but are simply held in place by gravity.3 Engineers have therefore been able to predict the behavior of structures, bearings, and cars by presuming that adhesion is zero. Yet it is evident that the small component parts of the cup, i. e. the grains and ultimately the atoms and molecules, stick together extremely well. Otherwise the cup would crumble to dust or would evaporate into the gas phase. Similarly your car would fall apart very easily, as my cars often do.
Therefore we have to decide what is the most fundamental nature of matter. Does it stick or not? We can short-cut to the answer given in Chapter 3. The first Law of Adhesion is that “All atoms adhere with considerable force.” This is as strange and surprising as the law of motion enunciated by Newton. How could our world operate if all the atoms were stuck fast and immobilized? Cars would not work because the tires would stick to the road. Indeed, the car engine parts would all seize together and stop moving. The paradox is that the car is made up of thousands of parts, most of which do stick together rather well. But some do not. This book sets out to explain this paradox.
First imagine what would happen to our planet if this first law of adhesion were taken at face value. The sands and soils which we grow our food on would stick together to form strong solid rocks. Sand grains would adhere so strongly that the beach would resemble sandstone. Soil would transform into mudstone, so tough that plants would not be able to push their roots through it and would perish. Any particles blown out of volcanoes would float around in the air until they touched a surface. Then they would stick immediately to that surface, making windows opaque and clogging machinery. The Earth’s atmosphere would be as clear and transparent as that on Mars. We know from the fossil record that sand beds can transform into sandstone and mud into mudstone, but this only happens over long times under extremes of pressure, temperature, or chemical modification. Clearly, something is retarding this adhesion under normal earthly conditions.
A clue to this problem comes from experiences of space travel.4 When the first lunar modules landed, dust did leap onto the windows, making them opaque. Furthermore, the solar panels became obscured by detritus which stuck firmly to the surfaces. Also, the hinges on the spacecraft doors did tend to seize up. This proved the idea that clean surfaces in the vacuum of space were much more adhering than the dirty, wet, oxygen-covered surfaces here on Earth. Thus we conclude that contamination of the surfaces is very important in reducing the atomic adhesion to low values, well below what we should expect from the first law of adhesion.
The second clue came from the study of electrical contacts and friction of bearings in the 1930s, as illustrated in Fig. 1.4. Holm5 and Bowden and Tabor6 realized that bodies apparently in contact were not truly touching over the geometric contact area. This was confirmed later by attempts to pass ultrasonic waves through contacts. The waves were inhibited at the contact, suggesting that only a small area of the bodies was really in molecular contact. It became clear that a design for an electrical contact could not be made on the basis of apparent
|
Figure 1.4. Three tests to show that contact is usually imperfect: (a) electrical test; (b) friction test, and (c) ultrasonic test. |
geometry. Perhaps one square centimeter of metal appeared to be in contact, whereas measurements revealed that only one square millimeter was actually passing electrical current. In the same way, the frictional force required to shear the contact was much smaller than expected. The ultrasonic transmission was also reduced.
Thus the paradox of adhesion being unpredictably large or small can be explained by these two complexities of contact at the molecular level; contamination and roughness.
 19 августа, 2015
19 августа, 2015  Pokraskin
Pokraskin 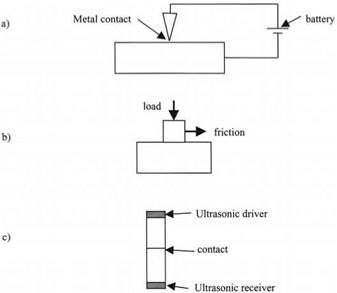
 Опубликовано в рубрике
Опубликовано в рубрике 