In adhesion science and technology, the manifestations of acid-base interactions have been observed at both macroscopic and microscopic scales (wetting, adhesion, metallization, etc.). The development of scanning probe microscopic methods (scanning tunneling microscopy (STM) and atomic force microscopy (AFM)) over the past decade has led to the possibility of measuring adhesion forces on the molecular scale in addition to imaging surfaces in atomic resolution.
In STM, atomically resolved images of the surface region are generated by bringing a metallic tip under piezoelectric control to within angstroms of a surface. At these small distances electrons can tunnel from the tip on application of a bias voltage. By using an electronic feedback system, one can keep the current (and hence the gap between tip and sample) constant as the tip is moved sideways across the surface. Because the current detection is so sensitive, the tip actually has to ride up over the atoms of the surface resulting in a ‘‘topographic’’ image of the surface.
The images generated from the variations in tunneling current are representative of differences in the local density of states across the surface. However, because STM relies on the conduction of electrons through the sample, it is generally not suitable for characterizing insulating samples such as organic polymers.
The atomic force microscope, an adaptation of the STM approach, can be used to measure interfacial forces with nanonewton sensitivity between the tip and a conducting or insulating surface in addition to topographic measurements. AFM monitoring of the long range attractive or repulsive forces between the tip and the sample surface permits elucidation of local chemical and mechanical properties such as adhesion and elasticity, and even thickness of adsorbed molecular layers. The forces that can lead to attractions of the tip to the surface include van der Waals interactions, hydrogen bonds, capillary action, or electrostatic fields. When the tip is brought near to the surface it may jump into contact in the case of sufficient attractive force. Once in contact, repulsive forces lead to a deflection of the cantilever in the opposite direction. Signals optically detected from either type of deflection (attractive or repulsive) can be used as the feedback signal. On withdrawal, adhesion during contact may cause the cantilever to adhere to the sample some distance past the initial contact. The tip becomes free from the surface at a point where adhesion is broken. The measured rupture force required to break adhesion is a key parameter of the AFM force curve.
In early studies, classical (tungsten or Si3N4) tips were found to be sensitive to the nature of the material surface. For example, adhesion forces between the tungsten tip and untreated and stearic acid-treated Al2O3/Al was much larger than between the tip and polytetrafluoroethylene (PTFE) most probably because the PTFE undergoes only dispersive interactions [177].
AFM has been used to measure the double layer interaction between the Si3N4 tip and a silica substrate [178]. The transition from the attractive to the repulsive double layer was found to be pH dependent. The double layer force measured at a distance of 17 nm is repulsive at pH >6.2 because the tip and the surface are both negatively charged. In contrast, the double layer interaction becomes attractive at pH <6.2, meaning that the tip and the silica have opposite signs. At pH = 6.2, the Si3N4 tip is not charged and hence the IEPS for the tip can be estimated by AFM.
Similar studies were aimed at measuring double layer forces between colloidal particles (silica, glass) glued to the tip and self-assembled monolayer-modified substrates. The rationale for using silica — or glass-modified tips is that silicon oxide has a low IEPS and bears a negative charge over a wide pH range. In contrast, the confined carboxylic acid is a weak Bronsted acid and can thus be either neutral at low pH or ionized at pH 5-6. This makes the study of electrostatic attractions and repulsions possible via AFM. In the case of interactions between carboxylic acid-terminated thiol and a glass-modified tip, it was found that below pH 6-6.3 the interaction was purely attractive (Fig. 20) because the terminal COOH groups were not ionized [179]. At higher pH, repulsive forces operate at the tip-COO “ interface. The force-to-distance curves showed a decrease in the repulsive component of the interaction as the electrolyte concentration was increased.
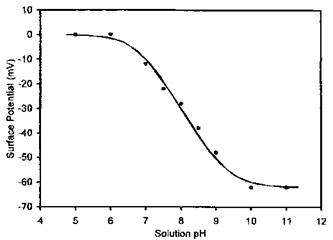
Hu and Bard [180] used a silica colloidal particle-modified tip to scan the surface of carboxylic acid-terminated thiols grafted onto gold substrate. They correlated the surface potential of the surface-confined COOH groups to the solution pH in which the substrate was immersed (Fig. 21). The sigmoidal shape of the plot suggests that surface potential is pH dependent. Since the surface potential is directly related to the fractional degree of surface carboxylic acid dissociation one can thus view the plot in Fig. 21 as a direct surface acid titration curve. It is noteworthy that the apparent pKa of the adsorbed COOH is near pH 8.0, much higher (about 3.5 units) than the pKa measured for similar acids in bulk aqueous solution. Similar studies using contact angle titration indicated such an increase in the apparent pKa of confined COOH groups as compared to the situation in the bulk solution [79]. This has been attributed to strong lateral hydrogen bonding between the confined COOH groups [180].
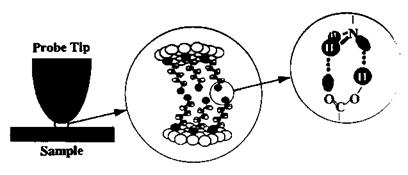
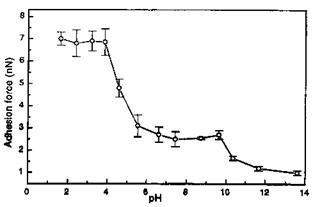
In order to systematically study the surface acid-base phenomena in a controlled manner, both tip and surfaces were functionalized by alkyl-, COOH-, PO3H2-, and NH2- terminated thiols (Fig. 22). In this regard, thiol-functionalized tips and surfaces were used to obtain adhesion force titration curves for carboxylic acid, phosphonic acid, and amino groups [182]. Figure 23 shows the study of the acid-base properties of a diprotic acid by the pH-dependent adhesion force measurement between a PO3H2-functionalized tip and PO3H2-functionalized surface. The overall pH-dependent behavior of the PO3H2 shows the ionization steps PO3H2 ! PO3H~ and PO3H~ ! PO3~ which correspond to effective pKa values of 4.7 and 11.6, respectively.
Thomas et al. [20] have measured adhesion forces between organic films over separations ranging from 10 nm to repulsive contact using interfacial force microscopy (IFM).
|
Figure 20 Normalized forces as a function of distance recorded between a 20 mm hydrophilic glass and a hydrophilic substrate in 3 x 10—4-7 x 10—4 M NaCl as a function of pH: (♦) 2.0; (□) 3.8; (+) 4.7; (O) 8.2; (—) 9.7. (Reprinted with permission of the American Chemical Society from Ref. [179], E. Kokkoli and C. F. Zukoski, Langmuir 16: 6029 (2000).) |
|
Figure 21 Measured (•) and theoretical (—) surface potentials of the carboxylic acid monolayer in 10—3 M KCl solutions at 25OC as a function of pH. The best theoretical fit gives surface pKa at pH 7.7. (Reprinted with permission of the American Chemical Society from Ref. [180], K. Hu and A. J. Bard, Langmuir 13: 5114 (1997).) |
|
Figure 22 Schematic illustration of scanning probe studies of (acid-base) hydrogen bonding between functional group terminated self-assembled monolayers of n-alkanethiol molecules. The thiol monolayers self-assemble on gold substrates, thus both tip and sample are initially coated with gold. (Reprinted with kind permission of VSP Publishers from Ref. [181], A. R. Burns, J. E. Houston, R. W. Carpick, and T. A. Michalske, in Ref. [19], Acid-Base Interactions: Relevance to Adhesion Science and Technology, Vol. 2 (K. L. Mittal, ed.), VSP, Utrecht, 2000, pp. 223-234.) |
|
Figure 23 ‘‘Adhesion force titration curve’’ for a PO3H2 functional tip on a PO3H2 functional substrate at constant ionic strength. (Reprinted, in part, with permission of the American Chemical Society from Ref. [182], E. W. van der Vegte and G. Hadziioannou, J. Phys. Chem. B 101: 9563 (1997).) |
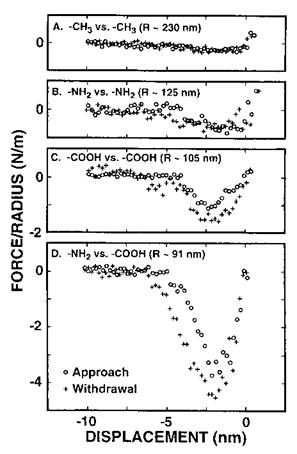
This microscope uses a self-balancing force-feedback system to avoid the mechanical instability encountered in AFM or STM, due to the use of deflection-based force sensors. The quantitative measure of the adhesion forces between organic films was achieved by chemically modifying a gold substrate and a gold tip with organomercaptan self — assembled monolayers (SAMs) having either the same or different end groups (-CH3, — NH2, and — COOH). Figure 24 shows representative force profiles for some terminal group combinations. The arbitrary zero displacement represents the point where the interaction force goes through zero while the probe is in contact with the sample. The force axis is normalized to the probe radius and the same scale is used for direct comparison of the different chemical interactions. The peak value of the attractive force from the unloading
|
Figure 24 Force versus displacement curves taken between (A) two methyl-terminated SAMs, (B) two amine-terminated SAMs, (C) two carboxylic acid-terminated SAMs, and (D) an amine — and a carboxylic acid-terminated SAM. The force is normalized to R, the tip radius. (Reprinted with permission of the American Chemical Society from Ref. [20].) |
curve (pull-off force) was used to evaluate the work of adhesion, W, for the various combinations using W = F/2nR, where R is the tip radius. The W values were found to be 60 ± 32, 100 ± 24, 228 ± 54, and 680 ± 62mJ/m2 for the CH3 versus CH3, NH2 versus NH2, COOH versus COOH, and NH2 versus COOH combinations, respectively. These values qualitatively scale with those expected for van der Waals, hydrogen-bonding (for NH2 versus NH2 and COOH versus COOH pairs) and acid-base interactions. In the last case, the interfacial energy was found to be large and negative, and corresponded to a NH2-COOH bond energy of 67kJ/mol. High work of adhesion was obtained for such dissimilar materials.
 25 июня, 2015
25 июня, 2015  Malyar
Malyar 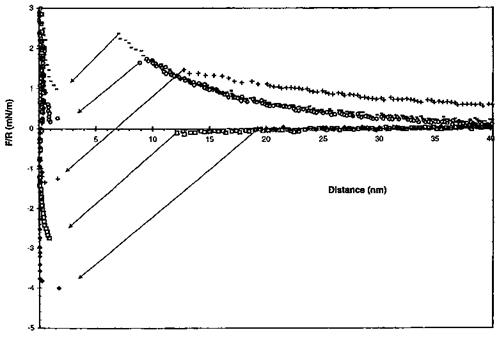
 Опубликовано в рубрике
Опубликовано в рубрике 