The water-water hydrogen bond is, for example, responsible for the anomalously high boiling point of water and contributes to 70% of the surface tension of this liquid at ambient temperature. It is also well known that the hydrogen bonds between complementary base pairs thymine-adenine (two bonds) and cytosine-guanine (three bonds) are the key to the double helical stucture of DNA. Finally, it has long been recognized that acid-base interactions have a dramatic effect on polymer macroscopic properties such as glass transition temperature [24], polymer miscibility [25,26], solubility in common solvents [14,27], swelling [27], adsorption [11], and adhesion [12,19].
|
Table 1 Types of Lewis Acids and Bases Electron Donors (bases) Electron Acceptors (acids)
|
|
Figure 3 Variation of the intermolecular distance X at the interface versus the reversible work of adhesion W. |
Hydrogen or acid-base bonds are exothermic and their energy ranges from 8-50kJ/mol [28,29]. This is comparable with London forces but exceeds dipole-dipole (Keesom) and dipole-induced dipole (Debye) interactions. With a large and negative value, the heat of an acid-base interaction can overcome the positive or the negligibly small negative entropic term —TAS, so that adhesion and mixing can be substantially improved. The high energy associated with acid-base interactions is due to Coulombic forces acting at intermolecular distances of ca. 0.2-0.3 nm. Acid-base interactions are thus of the short range type by comparison to the long range London dispersive interactions which can operate at distances exceeding 10 nm. For example Nardin and Schultz [29] have demonstrated for a series of single-fiber composites that the maximal work of adhesion (W) was obtained for fiber-matrix systems interacting via both dispersive and acid — base interactions on the one hand and for the smallest intermolecular distance X of ca. 0.2 nm on the other hand (see Fig. 3). For such a short distance, the highest heat of acid-base interaction (ca. 50 J/mol) between the fiber and the matrix was obtained.
The importance of acid-base interactions in various fields of chemistry led to extensive research in the 1960s to obtain acid-base scales. This resulted in the Hard and Soft Acids and Bases (HSAB) scales of Pearson [30], Drago’s E and C constants [31], and Gutmann’s donor and acceptor numbers [32]. Bolger and Michaels [33] have used Bronsted acid-base chemistry to predict the adhesion of organic and inorganic species.
 22 июня, 2015
22 июня, 2015  Malyar
Malyar 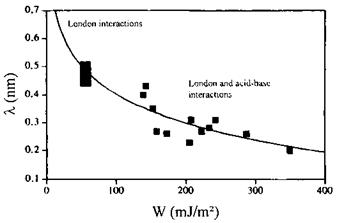
 Опубликовано в рубрике
Опубликовано в рубрике 