Adhesion of thermodynamically incompatible polymers is of current interest because of its implications for developing new multiphase polymer materials and for recycling of mixed plastic wastes. Many elegant experiments have been reported in which various types of copolymer are introduced at the interface as putative compatibilizers. The interface may be strengthened as a result of interdiffusion and roughening on a nanoscale.
A number of these experiments use the surface forces apparatus [79,80] in which extremely sensitive measurements of the force-distance characteristics can be made as surfaces of defined geometry, such as crossed cylinders or a sphere and a plane, are brought into contact and then separated. From these measurements a value of the interfacial energy of the two materials can be derived.
Creton et al. [58] studied the adhesion of a system somewhat similar to Hashimoto’s discussed above, using a surface forces-type apparatus. Contact was made between a cross-linked polyisoprene hemisphere and a thin polystyrene sheet. Under these circumstances, the fracture energy was low, comparable in magnitude (although not numerically close) to the work of adhesion 0.065 J/m2. However, when the polylstyrene surface was covered with a layer of a styrene-isoprene diblock polymer considerably higher adhesion was observed which increased with crack speed. The limiting value at zero crack speed, G0 increased with both surface density, £, and degree of polymerization, NPI, of the polyisoprene chains (Fig. 7). While the blurring of the interface is on a much more limited scale than that shown by Hashimoto, Creton et al. argue that the isoprene end of the diblock copolymer molecules diffuses into the cross-linked polyisoprene, and that the additional fracture energy is associated with the frictional drag as these chains are pulled out under the influence of the applied load.
With suitable copolymers, roughening of the interface between two incompatible polymers by interdiffusion can lead to a range of values for fracture toughness G. For diblock copolymers both surface density (£) and degree of polymerization (N) of the blocks are important. If the blocks are shorter than the entanglement length Ne of the corresponding homopolymer, failure occurs, as with the isoprene above, by chain pull-out and G is low. If N> Ne chain scission will occur at low surface density (£), but as £ is increased the fracture energy G rises steeply and plastic deformation, for example crazing, occurs in the polymer followed by chain scission or pull-out.
These effects have been found by Creton et al. [81] who laminated sheets of incompatible polymers, poly(methyl methacrylate) (PMMA) and poly(phenylene oxide) (PPO), and studied the adhesion using a double cantilever beam test to evaluate fracture
|
Figure 7 Increase in threshold fracture energy, G0, with length, NPI, and surface density, E of isoprene chains (after Ref. 58). Degree of polymerization: (I) 558, (II) 882, (III) 2205. |
|
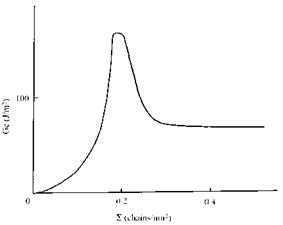
Figure 8 Adhesion of PMMA to PPO. Effect on fracture toughness, Gc, of interfacial density, E, of a reinforcing diblock copolymer (after Ref. 81). |
toughness Gc. For the original laminate Gc was only 2J/m2, but when the interface was reinforced with increasing amounts of a symmetrical PMMA-polystyrene diblock copolymer of high degree of polymerization (N > Ne), the fracture toughness increased to around 170 J/m2, and then fell to a steady value of 70 J/m2 (Fig. 8).
At low surface coverage fracture occurs close to the junction point of the diblock, with each fragment remaining on the ‘‘correct’’ side of the interface. At higher values of E the surface saturates, crazing occurs during fracture, and Gc reaches a maximum. With further increase in surface density of the copolymer a weak layer forms at the interface and the fracture toughness falls to a limiting value.
Toughening of a polymer-polymer interface with random copolymers may be more effective than with diblocks, when polymers are not too incompatible [82]. This is of industrial, as well as of scientific, interest as random copolymers are usually cheaper to produce.
Diblock copolymers will form a single, strong chemical linkage across the interface, but a random copolymer—if incompatability not too large—will form Gaussian coils wandering many times across the interface. If the incompatibility is too large the copolymer will simply form collapsed globules at the interface, forming a weak boundary layer giving no enhancement of adhesion.
 21 июня, 2015
21 июня, 2015  Malyar
Malyar 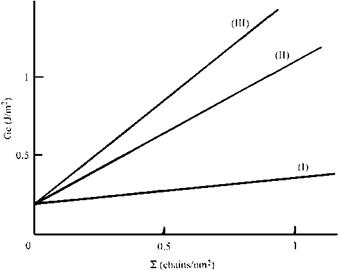
 Опубликовано в рубрике
Опубликовано в рубрике 