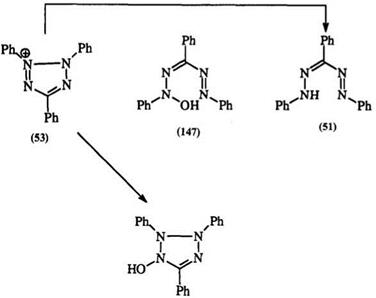
Tetrazolium salts are unstable in basic solutions yielding intense colors. This reaction is still little understood.233,234 In the reaction of 2,3,5- triphenyltetrazolium with hydroxide, it is postulated that a hydroxide ion is involved first as a counterion later leading to the hypothetical A-hydroxy — formazan (147).229 Weiner studied the kinetics of this reaction and identified 1,3,5-triphenylformazan in 10% yield. In concentrated alkaline solutions, the A-hydroxytetrazole (148) has been isolated from triphenyltetrazolium chlor — ide(Scheme 19).235-236
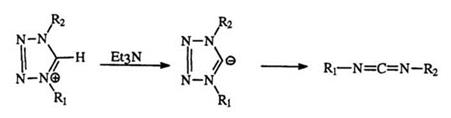
The reaction of 1,4-disubstituted tetrazoliums (149) with aliphatic tertiary amines such as triethylamine leads via deprotonation to 150 followed by ring opening to form a carbodiimide (74) with the loss of nitrogen (Scheme 20).237,238 Under the same conditions, 1,4,5-trisubstituted
|
|
|
(151)
R1,R2 = Alkyl or Aryl
R3 = Alkyl
|
|
|
|
|
|
RjR = Alkyl or Aryl
Scheme 20
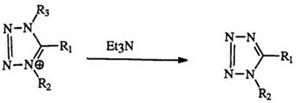
tetrazoliums (151), on the other hand, undergo dealkylation yielding 1,5-di — substituted tetrazoles (79) (Eq. 23).239
 24 сентября, 2015
24 сентября, 2015  Malyar
Malyar  Опубликовано в рубрике
Опубликовано в рубрике 