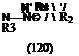 |
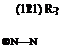 |
Nineham1 proposed that tetrazolium salts exist as resonance hybrids forms (120) and (121) (shown for 2,3-substituted ones). However, UV-
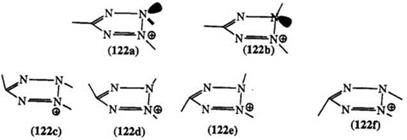 |
visible, IR, NMR, and differential calorimetric studies on unsymmetrically substituted 2,3,5-triaryl tetrazolium salts (with a substituted phenyl in the 5-position) indicate the existence of distinct isomers.197-206 Inversion at the trisubstituted nitrogen may be slowed down by the bulky aryl substituents on the adjacent nitrogen and the carbon, thus allowing conformational isomerism to occur, 122a and 122b.2 Schiele and co-workers proposed the possibility of four stereoisomers 122c to 122f for tetrazoliums with different substituents at N2 and N3200,203 but the exact mechanism of the isomerization is not clear, and has received little or no attention since first reported. Contrary to the resonance model, molecular orbital calculations using CNDO approximations show unequal electron densities on the nitrogens.207,208 It is not clear that this nonequivalence, in addition to the
unequal twist of the substituents on the 2- and 3-positions (Section 7.4.1.2), can account for the isomerism.
 19 сентября, 2015
19 сентября, 2015  Malyar
Malyar  Опубликовано в рубрике
Опубликовано в рубрике 