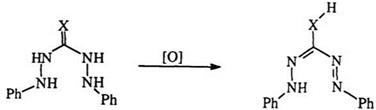
Diaryl carbazides and thiocarbazides, e. g., 43, can be oxidized at alkaline pH to yield C-hydroxy or C-mercapto formazans (44) (Eq. 8).68,129 The replacement of C-halo formazans is considered to be a better method60 for the preparation of 44. A class of cyclic formazans (45) can be obtained by air oxidation of amidrazones (46) (Eq. 9).69,73,101,102 Though this is formally a formazan, there are no reports of its oxidation reactions. In a
(8)
![]()
![]()
![]()
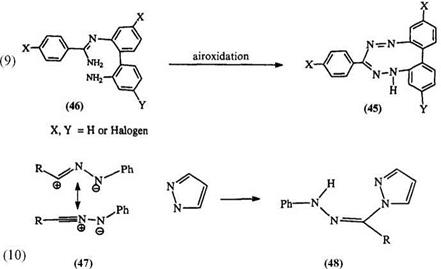 |
related reaction, nitrilimines, e. g., 47, which can be conveniently generated by the action of lead tetraacetate on aldehyde hydrazones, react with pyrazole to form the aryl hydrazone of 1-acyl pyrazole (48), a structure akin to formazan (Eq. 10).71,72
7.3.1.5. Oxidation of Formazans
A variety of oxidizing agents have been used to effect the transformation of formazans to tetrazolium salts. The choice of the reagent is still empirical and depends on the solubility properties of the formazan as well
as the resulting tetrazolium salt. The ease of oxidation is also influenced by the nature of the substituent.
The earliest oxidations were effected with “nitrous fumes” and later with mercuric oxide and isoamyl nitrite.74 Lead tetraacetate in acetic acid is in many cases the reagent of choice, but the removal of by-products can present some difficulties.75 N-Haloimides and amides in alcoholic solutions have been reported to yield essentially pure tetrazolium salts76 and have been found specially useful in the preparation of heteroaryl-substituted tetrazolium salt.77’78 The novel formazans 49 have been successfully oxidized to 50 using N-chloro succinimide (Eq. 11).79 tert-Butyl hypo-
|
(11) (49) (50) |
X = (CH2)n n= 1-6
R = H or CN
chlorite in chloroform or dioxane has also been reported to give good yields of tetrazolium Salts.80 Chlorine81 and bromine82 have been reported to be effective oxidizing agents, although nuclear halogenation on aromatic rings can occur in some cases. Lead sesquioxide,83 tribromophenol-bromine,84 and ammonium peroxodisulfate85 have also been reported. Hydrogen peroxide is generally ineffective, but can be very efficient in the presence of a catalyst such as ferrous ions or vanadium pentoxide.87-90 Recently, oxidations under phase transfer catalysis conditions have been studied and show great promise with potassium permanganate and nitrous acid.91 — 93 Many formazans are air sensitive and in some cases aerobic oxidation can be used as a preparative method of tetrazolium salts.31,58,95
Electrochemical oxidation has also been reported to result in good yields of tetrazolium salts.97,98 Treatment of formazans with boron trifluor-
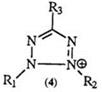 |
Table 3. Synthesis of Tetrazoliums from Oxidation of Formazans
|
Oxidizing
|
ide etherate86 or thionyl chloride94 has also been reported to yield tetrazoliums. Photooxidation has been suggested as a preparative method.96 This will be discussed in Section 7.5.2.5. Table 3 illustrates the use of various oxidizing agents in the synthesis of tetrazoliums.
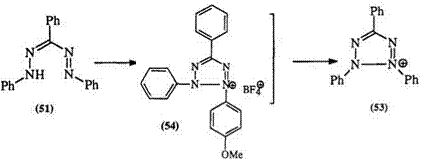
Wedekind and Stauwe99 studied the oxidation of 3-substituted forma — zans and concluded that ease of oxidation depended on the steric effects of the 3-substituent. More recently, Hegoraty et al. 100 studied the reaction of formazans with bromine. It proceeds via an odd-electron species such as 52 favoring an electronic substituent effect (Scheme 5). The rate of reaction increases with electron-donating substituents. Similar conclusions have been reached using thalium(III) as the oxidant.101’102
![]()
 |
of 2-(4-Methoxyphenyl)-3,5-diphenyltetrazolium tetrafluoro — To a stirred solution of 7.0 g of 1-(4-methoxypheny1)-3,5-
diphenylformazan in 400 ml of ethyl acetate was added a solution of 8.0 g of A-bromosuccinimide in 200 ml of ethyl acetate. The solution became colorless followed by partial precipitation. The yellow oily mass solidified on decantation of the solvent. It was taken up in 1500 ml boiling water, cooled, and filtered. The tetrazolium salt was precipitated by the addition of 200 ml 2 M sodium tetrafluoroborate. The off-white crystals were recrystallized from acetonitrile/ether, filtered, and air dried to yield 6.1 g (70%) of the tetrazolium salt.
 15 сентября, 2015
15 сентября, 2015  Malyar
Malyar 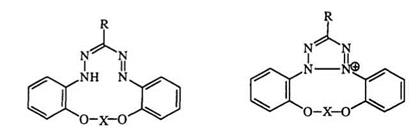
 Опубликовано в рубрике
Опубликовано в рубрике 