Treatment of 2′-anilinofluorans with ketones such as acetone or 2- butanone in hydrochloric acid in the presence of iron(III) chloride gives 4,4′-alkylidenebis(N-fluoran-2-ylaniline)s. Thus, 2′-anilino-6′-(N-cyclohexyl- N-methylamino)-3′-methylfluoran, (86) is treated with acetone in hydrochloric acid in the presence of iron(III) chloride to give 2,2-bis(4-[6′-(N- cyclohexyl-N-methylamino)-3′-methylfluoran-2′-ylamino]phenyl}propane (87)60 (Eq. 9).
|
|
(9)
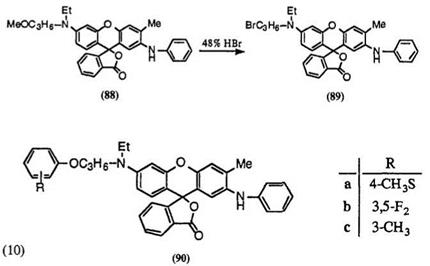
Treating 2′-anilino-6′-[N-ethyl-N-(3-methoxypropyl)amino]-3′-methyl-
fluoran (88) with 48% hydrobromic acid in the presence of concentrated sulfuric acid at 110-11 5 °C gives 2′-anilino-6′-[N-(3′-bromopropyl)-N — ethylamino]-3′-methylfluoran (89)70 in excellent yield (Eq. 10).
The fluoran 89 reacts with phenols in N, N-dimethylacetamide in the presence of potassium carbonate to give 2′-anilino-6′-( N-ethyl-N-[3-(4- methylthiophenoxy)propyl]amino}-3′-methylfluoran (90a),71 2′-anilino-6′-
{N-ethyl-N-[3-(3,5-difluorophenoxy)propyl]amino}-3′-methylfluoran (90b),71 2′-anilino-6′-(N-ethyl-N-[3-(3-methylphenoxy)propyl]amino}-3′- methylfluoran (90c),71 etc. in excellent yield.
 |
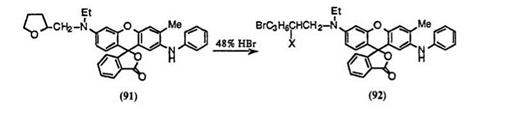
Tetrahydrofuran moiety of 2′-anilino-6′-(N-ethyl-N-tetrahydrofurfur — ylamino)-3′-methylfluoran (91) can be hydrolyzed with 48% hydrobromic acid in sulfolane to give 2′-anilino-6′-[N-(5-bromo-2-hydroxypentyl)-N- ethylamino]-3′-methylfluoran (92; X = OH)72 and 2′-anilino-6′-[N-(2,5-di — bromopentyl)-N-ethylamino]-3′-methylfluoran (92; X = Br)72 at 70-90 °C and 100 °C, respectively (Eq. 11).
(11)
Preparation of 2,2-Bis(4-[6′-(N-cyclohexyl-N-methylamino)-3′-methyl- fluoran-2′-ylamino]phenyl)propane (87). To a solution of 2′-anilino-6′-(N- cyclohexyl-N-methylamino)-3′-methylfluoran (0.1 mol) in 250 ml of acetone heated at 60°C was added dropwise 300ml of 35% hydrochloric acid over a period of 30min, and stirring was continued for 30min. To this, after being cooled to room temperature, was added iron(III) chloride (0.04 mol). The resulting mixture was stirred at room temperature overnight, diluted with 3 liters of water, and neutralized by sodium bicarbonate. The precipi
tate was filtered off, and recrystallized from toluene in the usual manner to give 2,2-bis{4-[6′-(N-cyclohexyl-N-methylamino)-3′-methylfluoran-2′-yl- amino]phenyl}propane in 35% yield as a white powder, mp 237-239 °C.
Preparation of 2′-Anilino-6′- [N- (3-bromopropyl) — N-ethylarnino]-3′- methylfluoran (89). To a mixture of 2′-anilino-6′-[N-ethyl-N-(3-methoxy — propyl)amino]-3′-methylfluoran (0.1 mol) and 48% hydrobromic acid (150 ml) was added dropwise concentrated sulfuric acid (20 ml) with vigorous stirring. Then, the resulting mixture was stirred at 110-115 °C for 1 h, poured into ice water (1000ml), and made alkaline by aqueous sodium hydroxide. The pale violet precipitate was filtered off, and recrystallized from ethyl acetate-isopropanol to give 2′-anilino-6′-[N-(3-bromopropyl)- N-ethylamino]-3′-methylfluoran in 96% yield, mp 160-162 °C.
Preparation of 2 ‘-Anilino-6′-(N-ethyl-N-[3-(4-methylthiophenoxy)pro- pyl]amino}-3′-methylfluoran (90a). A mixture of 2′-anilino-6′-[N-(3-bro- mopropyl)-N-ethylamino]-3′-methylfluoran (0.1 mol), 4-(methylthio)phenol (0.1 mol), and potassium carbonate (0.14 mol) in N, N-dimethylacetamide (100ml) was stirred at 85 °C for 1 h. The reaction mixture was poured into ice water (500ml). The precipitate was filtered off, and recrystallized from ethanol to give 2′-anilino-6′-{N-ethyl-N-[3-(4-methylthiophenoxy)propyl]- amino}-3’-methylfluoran in 91% yield, mp 187-189 °C.
 5 сентября, 2015
5 сентября, 2015  Malyar
Malyar 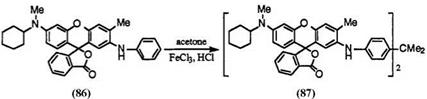
 Опубликовано в рубрике
Опубликовано в рубрике 