6.2.2. Reaction of Keto Acids with Phenols
The reaction of keto acids with phenols is mainly used to prepare fluoran compounds developing colors from orange to red.
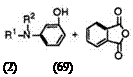
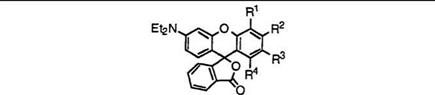
That is, keto acids (66) react with a wide variety of phenols (67) to give 3′-aminofluorans (68) (Eq. 1).
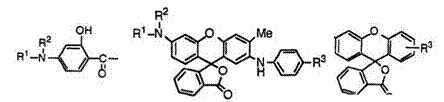 |
Table 1. Crystal Modifications of Fluoran Compounds
|
R1 |
R2 |
R3 |
mp a-form |
°C P-form |
Ref. |
|
CH3 |
n-C3H7 |
H |
175-177 |
178-181 |
66 |
|
C2H5 |
n-C4H9 |
H |
162-164 |
181-183 |
67 |
|
n-C4H9 |
n-C4H9 |
H |
148-152 |
180-184 |
52 |
|
n-C4H9 |
n-C4H9 |
F |
135-137 |
169-171 |
68 |
|
n-C5H11 |
c-C6Hn |
H |
136-141 |
168-171 |
67 |
 |
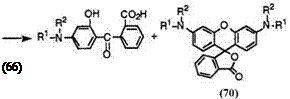 |
The keto acids (66) having a tertiary amino group at 4-position are successfully prepared by the reaction of 3-terf-aminophenols (69) with phthalic anhydride in organic solvent such as benzene, toluene, or chlorobenzene at elevated temperature, though each keto acid has its own
characteristic reaction temperature, because some 3-tert-aminophenols can produce 3′,6′-diaminofluorans (70) as a by-product at higher reaction temperature (Eq. 2).
Table 2 shows melting points of representative examples of the keto acids (66) having a tertiary amino group at 4-position. 2-(4-Diethylamino-2- hydroxybenzoyl)benzoic acid (66b) is the most popular among the keto acids.
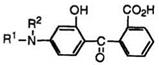
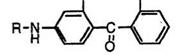
On the other hand, the reaction of 3-s’ec-aminophenols (71) with phthalic anhydride does not give the corresponding keto acids (72). The keto acids (72) having a secondary amino group at 4-position are prepared by the reaction of 3-s’ec-aminophenols (71) with phthalimide at 150-220°C in the presence of boric acid, followed by hydrolysis of the intermediate carboxamide with aqueous sodium hydroxide (Eq. 3).
|
|
Table 3 shows melting points of a few representative keto acids (72). The reaction of keto acids (66) with phenols (67) is usually carried out in 70-90% sulfuric acid at 50-150°C, or in an aromatic solvent such as benzene, toluene, or chlorobenzene at reflux in the presence
Table 2. Melting Points of Keto Acids (66)
|
|
|
66 |
R1 |
R2 |
О 0 її |
|
a |
CH3 |
CH3 |
185-186 |
|
b |
C2H5 |
C2H5 |
207-208 |
|
c |
C2H5 |
i-C4H9 |
141-142 |
|
d |
C2H5 |
i-C5Hii |
130-131 |
|
e |
n-C4 H9 |
n-C4H9 |
190-192 |
|
f |
CH3 |
C6H5 |
164-165 |
|
g |
CH3 |
4-CH3 C6 H4 |
201-202 |
|
h |
C2H5 |
C6H5 |
186-187 |
|
i |
C2H5 |
4-CH3 C6 H4 |
172-175 |
|
OH CO2H
|
|
72 |
R |
О 0 її |
|
a |
с-СбНії |
140-142 |
|
b |
C6H5CH2 |
190-192 |
|
c |
C6H5 |
184-186 |
|
d |
4-CH3 C6 H4 |
204-205 |
|
e |
4-C2H5C6H4 |
174-176 |
|
f |
4-w-C4H9C6H4 |
170-171 |
of a small amount of concentrated sulfuric acid. Many phenols such as p-cresol, 3,5-dimethylphenol, 2,3,5-trimethylphenol, p-chlorophenol, p — chloro-m-cresol, P-naphthol, or 6-bromo-P-naphthol give the corresponding 3′-aminofluorans (68) in good yield, though phenol and ornaphthol give lower yield. In the case of 1-bromo-P — naphthol, the bromine atom is liable to eliminate in acidic media resulting in the formation of the same fluoran compound derived from P-naphthol.
Table 4 shows results of a few representative 3 ‘-diethylaminofluorans (68; R1, R2 = C2H5) prepared by the reaction of 2-(4-diethylamino-2- hydroxybenzoyl)benzoic acid (66b) with phenols.
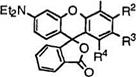
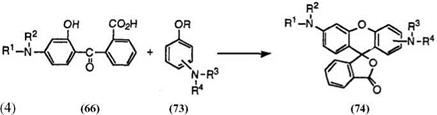
The keto acids (66) also react with aminophenols (73; R = H) or aminoanisoles (73; R = CH3) to give diaminofluorans (74) (Eq. 4).
|
|
In this case, it is preferable to carry out the reaction in concentrated sulfuric acid at up to 60 °C to achieve good results.
|
|
|
68 |
Ri |
R2 |
R3 |
R4 |
Yield (%) |
О 0 |
|
a |
H |
H |
CH3 |
H |
82 |
160-161 |
|
b |
CH3 |
CH3 |
H |
CH3 |
85 |
236-237 |
|
c |
C1 |
H |
CH3 |
H |
84 |
207-209 |
|
d |
H |
CH3 |
Cl |
H |
85 |
235-236 |
|
e |
H |
H |
C1 |
H |
84 |
174-176 |
|
f |
H |
H |
—CH=CH—CH=CH— |
90 |
220-221 |
|
|
g |
H |
H |
—CH=CBr—CH=CH— |
85 |
240-241 |
|
|
h |
О ffi |
CH—CH=CH— |
H |
H |
34 |
191-192 |
|
Table 5 shows results of a few representative of diaminofluorans (74) prepared by the reaction of the keto acid (66b) with aminophenols or aminoanisoles. 2 ‘-Amino-6 ‘-diethylaminofluoran (74a) is an important precursor for 2′-dibenzylamino-6’-diethylaminofluoran developing green color. |
Preparation of 2- (4-Diethylamino-2-hydroxybenzoyl) benzoic acid (66B). A mixture of 3-diethylaminophenol (1 mol) and phthalic anhydride (1.1 mol) in toluene (400 ml) was stirred at reflux for 5 h. After being cooled, the precipitate was filtered off, washed with methanol, and dried to give 2-(4-diethylamino-2-hydroxybenzoyl)benzoic acid in 90% yield as a pale pink powder, mp 207-208 °C.
Preparation of 2- (4-Di-n-butylamino-2-hydroxybenzoyl) benzoic acid (66e). A mixture of 3-di-n-butylaminophenol (1 mol) and phthalic anhydride (1.2 mol) in toluene (250 ml) was stirred at 65-70 °C for 24 h. After being cooled, the precipitate was filtered off, washed with toluene followed by methanol, and dried to give 2-(4-di-n-butylamino-2-hydroxy-benzoyl)- benzoic acid in 85% yield as a pale pink powder, mp 190-192°C.
Preparation of 2-[2-Hydroxy-4-(4-methylanilino)benzoyl]benzoic acid
(72d). To a molten mixture of 3-(4-methylanilino)phenol (1 mol) and
R1
|
74 |
Ri |
R2 |
R3 |
r4 |
Yield(%) |
О 0 % |
|
a |
H |
H |
nh2 |
H |
90 |
213-215 |
|
b |
CH3 |
H |
nh2 |
H |
73 |
175-178 |
|
c |
H |
C1 |
c2h5oc2h4nh |
H |
70 |
188-190 |
|
d |
H |
H |
n-C8H17 NH |
H |
75 |
127-128 |
|
e |
H |
H |
c6hch2nh |
H |
65 |
168-169 |
|
f |
H |
H |
—CH=C(NH2)—CH=CH— |
86 |
243-244 |
|
|
g |
—CH= |
=CH—CH=CH— |
nh2 |
H |
46 |
227-229 |
 |
phthalimide (1 mol) heated at 150°C was added boric acid (2 mol). The resulting mixture was stirred at 200-210 °C, while water formed was distilled out. The mixture solidified within 1 h. The solidified mixture was then heated with 10% aqueous sodium hydroxide (3500ml) for 4h to hydrolyze the carboxamide; a clear solution was formed followed by deposition of a new precipitate or sodium salt of the keto acid. The precipitate was filtered off, washed with water, dispersed in water (3000 ml), and made pH 4 by hydrochloric acid. After being refluxed for 15min, the solid part was filtered off, washed with hot water, dried, and then recrystallized from toluene/methanol (1:1) to give 2-[2-hydroxy-4-(4-methylanilino)- benzoyl]benzoic acid in 50% yield as a pale brown-green powder, mp 204-205 °C.
Preparation of 6′-Diethylamino-1′,3′,4′-trimethylfluoran (68b). A mixture of 2-(4-diethylamino-2-hydroxybenzoyl)benzoic acid (0.1 mol), 2,3,5- trimethylphenol (0.1 mol), and 90% sulfuric acid (150 g) was stirred at 50-55°C for 5 h, and poured into water (1500ml). The precipitate was filtered off, washed with water, and then refluxed with a mixture of toluene (400ml) and 5% aqueous sodium hydroxide (200ml) for 30min. The toluene layer was separated, washed with hot water, concentrated, and the residue refluxed with methanol (200 ml) for 30 min. After being cooled, the precipitate was filtered off, washed with methanol, and dried to give 6′-
diethylamino-1 ‘,3′,4’-trimethylfluoran in 85% yield as an off-white powder, mp 236-237 °C.
Preparation of 2′-Chloro-6′-diethylarnino-3′-methylfluoran (68d). A mixture of 2-(4-diethylamino-2-hydroxybenzoyl)benzoic acid (0.1 mol), p — chloro-m-cresol (0.1 mol), and 85% sulfuric acid (150 g) was stirred at 110-120°C for 5 h, and poured into water (1500ml). The precipitate was filtered off, washed with water, and then refluxed with a mixture of toluene (500ml) and 5% aqueous sodium hydroxide (200ml) for 1 h. The toluene layer was then worked up in the same manner as above to give 2′-chloro-6′- diethylamino-3 ‘-methylfluoran in 85% yield as a white powder, mp 235236 °C.
Preparation of 9′-Diethylaminobenzo[a]fluoran (68f). A mixture of 2-(4-diethylamino-2-hydroxybenzoyl)benzoic acid (0.1 mol) and P-naphthol (0.1 mol) in toluene (200 ml) containing concentrated sulfuric acid (5 ml) was stirred at reflux for 3 h, while water formed was removed as an azeotropic mixture with toluene. The mixture was diluted with toluene (200ml), made alkaline by 10% aqueous sodium hydroxide, and refluxing continued for another 1 h. The toluene layer was then worked up in the same manner as above to give 9′-diethylaminobenzo[a]fluoran in 90% yield as a white powder, mp 200-201 °C.
Preparation of 2′-Amino-6′-diethylaminofluoran (74a). A mixture of 2-(4-diethylamino-2-hydroxybenzoyl)benzoic acid (0.1 mol), p-anisidine
(0.1 mol), and concentrated sulfuric acid (100 g) was stirred at 50 °C for 24 h. After being cooled, the mixture was poured into water (500 ml), and made alkaline by 20% aqueous sodium hydroxide. The precipitate was filtered off, washed with water, dried, and then recrystallized from toluene to give 2′-amino-6′-diethylaminofluoran in 90% yield as an off-white powder, mp 213-215 °C.
Preparation of 6′-Diethylamino-2′-n-octylaminofluoran (74d). To a solution of 2-(4-diethylamino-2-hydroxybenzoyl)benzoic acid (0.1 mol) in 98% sulfuric acid (150 g) was added p-n-octylaminoanisole (0.1 mol) in limited amounts, while the temperature was maintained not to rise above 30 °C. The resulting mixture was stirred at room temperature for 20 h, and poured into ice water (1000 ml). The resinous precipitate was collected by decantation, and then refluxed with a mixture of toluene (250ml) and 20% aqueous sodium hydroxide (100 g) for 1 h. The toluene layer was separated, washed with hot water, concentrated, and the residue recrystallized from isopropanol (100 ml) to give 6′-diethylamino-2′-n-octylaminofluoran in 75% yield as a white powder, mp 127-128 °C.
 31 августа, 2015
31 августа, 2015  Malyar
Malyar 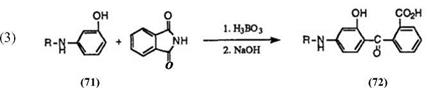
 Опубликовано в рубрике
Опубликовано в рубрике 