Fluoran compounds having two diarylamino groups at 3′- and 6′- positions generally develop blue tone colors. For example, 3′,6′-bis — (diphenylamino)fluoran (34; R1, R2 = H)29 develops reddish blue color, and 3′-diphenylamino-6′-di-p-tolylaminofluoran (34; R1 = H, R2 = CH3)29 and 3′,6′-bis(di-p-tolylamino)fluoran (34; R1, R2 = CH3)29 blue color.
|
(34) |
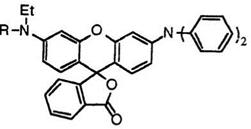
If at least one of the aryl groups at 3′-position is replaced with an alkyl group, then the developed color is more reddish as a result of a hypso — chromic shift. Thus, 3′-diethylamino-6′-diphenylaminofluoran (35; R = C2H5)29 and 3 ‘-diphenylamino-6 ‘-(N-ethyl-p-toluidino)fluoran (35; R = 4- CH3C6H4)29 develop reddish violet and bluish violet colors, respectively.
|
(35) |
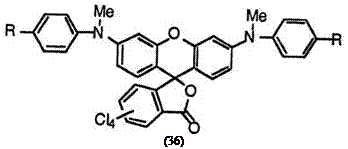
However, introducing halogens on the benzene ring of the phthalide moiety contributes a bathochromic shift. Thus, 3′,6′-bis(N-methylanilino)- 4,5,6,7-tetrachlorofluoran (36; R = H)30 and 3′,6′-bis(4-chloro-N-
methylanilino)-4,5,6,7-tetrachlorofluoran (36; R = C1)30 still develop purplish blue and blue colors, respectively.
 |
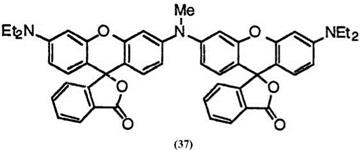 |
In addition, 3′,3m-N-methyliminobis(6′-diethylaminofluoran) (37)31 develops reddish blue color, in spite of no halogen atoms on the phthalide moiety.
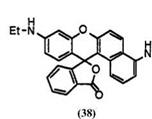
Some benzofluorans having two amino groups also develop blue color. These include 9′-ethylamino-4′-(methoxycarbonylmethylamino)benzo[a]-
fluoran (38),32 9′-diethylamino-4′-a-naphthylmethylaminobenzo[a]fluoran
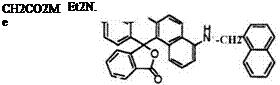 |
 |
(39),32 4′-cinnamylamino-10′-diethylaminobenzo[c]fluoran (40),32 and 10′- diethylamino-3′-(N-ethylanilino)benzo[c]fluoran (41).33
 27 августа, 2015
27 августа, 2015  Malyar
Malyar 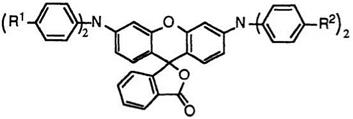
 Опубликовано в рубрике
Опубликовано в рубрике 