 |
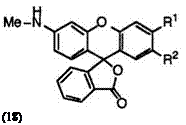 |
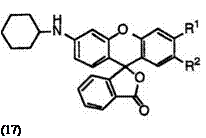
Fluoran compounds having a monosubstituted amino group at 3′-position generally develop orange color. Cyclohexyl group is most desirably used as the substituent on amino groups, though linear alkyl and aralkyl groups are also employed to give orange color. These include 2′-chloro-6′- cyclohexylaminofluoran (17; R1 = H, R2 = Cl),4 3′-chloro-6′-cyclohexyl — aminofluoran (17; R1 = Cl, R2 = H),14 6′-cyclohexylamino-2′-methylfluoran (17; R1 = H, R2 = CH3),4 2′-chloro-6′-methylaminofluoran (18; R1 = H, R2 = Cl),4 and 3′-chloro-6′-methylaminofluoran (18; R1 = Cl, R2 = H).13 Chlorine at 2′-position produces a smaller bathochromic shift than that at 3′-position.
In addition, 3′-(N-cyclohexyl-N-methylamino)-6′-methylfluoran (19)4 develops orange color, whereas fluoran compounds having a disubstituted amino group at 3′-position generally develop red color as described in Section 6.2.2.3.
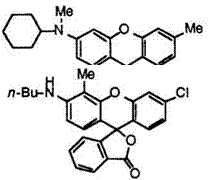
On the other hand, a methyl group adjacent to an amino group at 3′-position produces a bathochromic shift. Thus, 3′-chloro-6′-cyclohexyl — amino-4′,5′-dimethylfluoran (20),14 3′-«-butylamino-6′-chloro-4′-methylflu — oran (21),15 etc. develop vermilion color.
 |
 |
(21) (22)
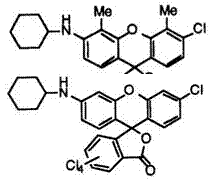
In addition, chlorine on the phthalide moiety also gives a bathochromic shift. Thus, 3′-cyclohexylamino-4,5,6,6′,7-pentachlorofluoran (22) 14 develops red color.
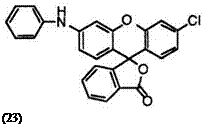 |
As a matter of course, replacement of (cyclo)alkylamino groups with arylamino groups will result in a bathochromic shift. For example, 3′- anilino-6′-chlorofluoran (23)13 develops yellowish red color.
6.2.2.3. Red Developing Fluorans
Fluoran compounds having a dialkylamino group at 3′-position generally develop color from yellowish red to vermilion. These include
 |
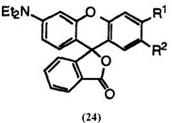 |
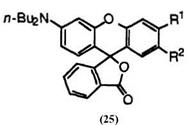
2′-chloro-6-diethylaminofluoran (24; R1 = H, R2 = C1), 16 2′-chloro-6′-dieth- ylamino-3′-methylfluoran (24; R1 = CH3, R2 = C1),17 6′-diethylamino-2′,3′- dimethylaminofluoran (24; R1, R2 = CH3),12 6′-diethylamino-2′-methoxy — fluoran(24; R1 = H, R2 = CH3O),12 3′-diethylamino-6′-methoxyfluoran (24 R1 = CH3O, R2 = H),18 6′-diethylamino-2′-phenylfluoran (24; R1 = H, R2 = C6H5),19 6′-diethylamino-2′-methylthiofluoran (24; R1 = H, R2 = CH3S),20 2′-chloro-6′-di-«-butylamino-3′-methylfluoran (25; R1 = CH3, R2 = C1),21 and 3′-chloro-6′-di-«-butylamino-2’methylfluoran (25; R1 = C1, R2 = CH3).21
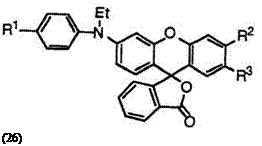 |
Fluoran compounds having an N-alkyl-N-arylamino group at 3′-posi — tion such as 2′-chloro-6′-(N-ethyl-4-methylanilino)fluoran (26; R1 = CH3, R2 = H, R3 = C1),22 6′-(N-ethyl-4-methylanilino)-2′-methoxyfluoran (26; R1 = CH3, R2 = H, R3 = CH3O),22 and 6′-(4-chloro-N-ethylanilino)-2′,3′- dimethylfluoran (26; R1 = Cl, R2, R3 = CH3)22 also develop vermilion color, but these color tones are more bathochromic because of longer conjugated double bond system.
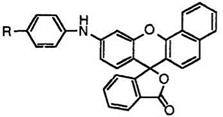
Benzofluoran compounds develop more bathochromic color than the corresponding fluoran compounds because of the longer conjugated system. Thus, 9′-diethylaminobenzo[a]fluoran (27; R1, R2 = C2H5),20 9′-(N-ethyl-
![]()
|
N-isopentylamino)benzo[a]fluoran (27; R1 = C2H5, R2 = /-C5HU),23 etc. develop bluish red color.
 |
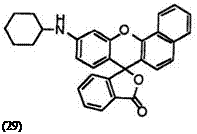 |
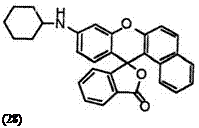
In addition, 9′-cyclohexylaminobenzo[a]fluoran (28),24 10′-cyclohexyl — benzo[c]fluoran (29),24 etc. develop red color, despite the secondary amino group that contributes generally orange color.
 |
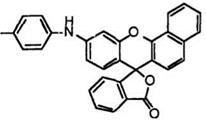 |
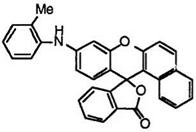
Benzofluoran compounds having an arylamino group develop much more bathochromic colors. Thus, 9′-(2-methylanilino)benzo[a]fluoran (30),24 10′-(4-methoxyanilino)benzo[c]fluoran (31),24 etc. develop violet color.
If the arylamino group is substituted with an appropriate group to extend the conjugated double bond system, blue color can also be obtained. For example, 10′-(4-anilinoanilino)benzo[c]fluoran (32; R = C6H5NH)24 and 10′-(4-styrylanilino)benzo[c]fluoran (32; R = C6H5CH=CH)25 develop blue color.
|
(32) |
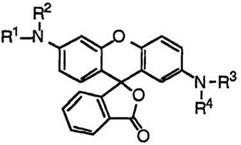
In addition, fluoran compounds having two amino groups at 2′- and 6′-positions develop red color when the 2′-amino group is an unsubstituted or acyl-substituted amino group. These include 2′-amino-6′— diethylaminofluoran (33; R1, R2 = C2H5, R3, R4 = H),5 2′-acetamino- 6′-diethylaminofluoran (33; R1, R2 = C2H5, R3 = H, R4 = CH3CO),26 2′— (N-acetylanilino)-6′-diethylaminofluoran (33; R1, R2 = C2H5, R3 = C6H5, R4 = CH3CO),27 2′-(N-benzoyl-N-methylamino)-6′-diethylaminofluoran (33; R1, R2 = C2H5, R3 = CH3, R4 = C6H5CO),28 and 2′-acetamino-6′-(N- methylanilino)fluoran (33; R1 = CH3, R2 = C,6H5, R3 = H, R4 = CH3CO).22
|
|
 25 августа, 2015
25 августа, 2015  Malyar
Malyar 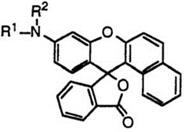
 Опубликовано в рубрике
Опубликовано в рубрике 