Fluoran compounds have the remarkable feature of giving a wide variety of colors depending on their substituent(s).
The following will discuss the effect of substituents for each developing color.
6.2.1.1. Yellow Developing Fluorans
Fluoran compounds having two alkoxy groups at 3′- and 6′-posi- tions develop yellow color, but the color intensity is not very high. These include 3′,6′-dimethoxyfluoran (14; R = CH3, X = H),10 2′,7′-dichloro-3′,6′- dimethoxyfluoran (14; R = CH3, X = Cl),10 3′,6′-diethoxyfluoran (14; R = C2H5, X = H),10 3′,6′-dibenzyloxyfluoran (14; R = C6H5CH2, X = H),10 3′,6′-bis(2-chloroethoxy)fluoran (14; R = C1C2H4, X = H),11 and 3′,6′-bis — (2-cyanoethoxy)fluoran (14; R = NCC2H4, X = H).11
|
|
Fluoran compounds having an unsubstituted amino group at 3’-position also develop reddish yellow color. These include 6′-amino-2′-f — butylfluoran (15; R1, R3 = H, R2 = f-C4H9),12 6′-amino-2′-f-butyl-3′- methylfluoran (15; R1 =CH3, R2 = f-C4H9, R3=H),12 6′-amino-1′,3′-di-
methylfluoran (15; R1, R3 = CH3, R2 = H),12 6′-amino-2′,3′-dimethylfluoran (15; R1, R2 = CH3, R3 = H),12 and 3′-amino-6′-chlorofluoran (15; R1 = Cl, R2, R3 = H)13.
On the other hand, if fluoran compounds have an unsubstituted amino group at 4-position such as 5′-amino-2′,3′,7′-trimethylfluoran (16),12 they develop greenish blue color.
 25 августа, 2015
25 августа, 2015  Malyar
Malyar 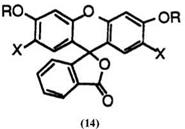
 Опубликовано в рубрике
Опубликовано в рубрике 