In our information-oriented society, computer and facsimile are being widely used as a means for transmitting information. Carbonless copying papers and thermosensitive recording papers, which utilize a color-formation reaction between leuco dyes and acidic compounds, have won high regard as recording papers used with these office machines. The leuco dyes are colorless or nearly colorless solids, but develop colors on contact with acidic compounds or electron-accepting compounds. Among various classes of leuco dyes, fluoran compounds have the remarkable feature of giving a wide variety of colors depending on their substituent(s). In particular, fluoran compounds are very important in their ability to yield singly black color which is hardly attained by the other classes of leuco dyes.
Fluoran (1) is the commonly used name for the spiro[isobenzofuran — 1,9′ — xanthen]-3-one. Benzo[a]fluoran (2) has the benzene ring fused to the 1- and 2-positions of the xanthene moiety. Fusion at the 3- and 4-positions gives benzo[c]fluoran (3). Numbering of the atoms is employed as shown in 1-3.
Fluoran compound used as leuco dye needs to have substituent(s) on the xanthene moiety to develop color, though fluoran 1 itself is prepared as a by-product in the synthesis of phenolphthalein from phenol and phthalic anhydride.
YOSHIHIRO HATANO • Research and Development, Yamamoto Chemicals, Inc., Yao, Osaka 581, Japan.
Chemistry and Applications of Leuco Dyes, edited by Muthyala. Plenum Press, New York, 1997.
|
|
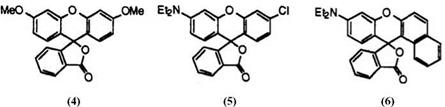
Fluoran compounds developing colors are not new, having been well known since early times. For example, the Beilstein Handbook of Organic Chemistry, XIX describes many fluoran compounds developing colors from yellow to red. These include 3′,6′-dimethoxyfluoran (4; yellow), 3′-chloro-6′- diethylaminofluoran (5; vermilion), and 9′-diethylaminobenzo[a]fluoran (6; red).
|
|
Fluoran compounds generally lack color stability, and therefore had lost their value as dyestuff for textile finishing. It is, however, very interesting that the old-fashioned fluoran compounds have come around as leuco dyes for use in the new applications.
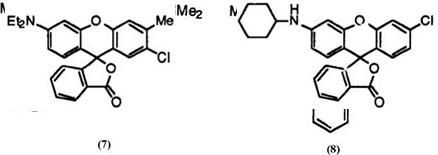
In 1954, the National Cash Register Company first marketed carbonless copying paper—NCR paper1—using Crystal Violet lactone (7)1 and benzoyl leuco Methylene Blue (8)2 as leuco dyes to make blue images.
In order to develop new leuco dyes, a great number of studies subsequently concentrated on fluoran compounds particularly in Japan, where there were high demands for carbonless copying papers developing black color. It was, however, not until the 1970s that black developing fluoran compounds became available. The first stage was, therefore, to produce orange or yellowish red developing fluorans such as 2′-chloro-
6
 |
‘-diethylamino-3 ‘-methylfluoran (9),3 3′-chloro-6’-cyclohexylaminofluoran (10),4 etc., to make mixed black color together with 7.
(9) (10)
The mixed black was, however, not very practical, because 7 is very high in saturation resulting in an unbalanced color tone.
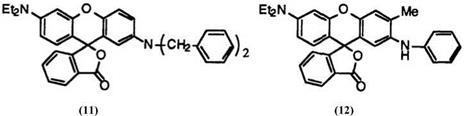
It was then that a very important invention that cannot be exaggerated for the development of fluoran chemistry was made, namely, the invention of 2′-dibenzylamino-6′-diethylaminofluoran (11)5 developing dark green color. Regarding dye chemistry, it was very surprising that such a small molecule as fluoran 11 develops green color. Fluoran 11 gives practical black color together with a red developing leuco dye, which mixed black color is even today used for carbonless copying papers employed inorganic coreactants.
A singly black developing leuco dye was ultimately realized by the invention of 2′-anilino-6′-diethylamino-3′-methylfluoran (12).6 Fluoran 12 skillfully utilizes the steric hindrance of a methyl group’ at 3′-position to develop black color (see discussion below). Practically all black developing fluoran compounds marketed today are derivatives of 12, though each has an individual characteristic, especially for use in thermosensitive recording papers.
|
|
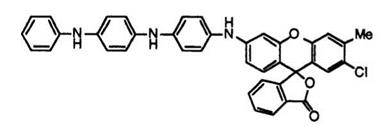
In addition, fluoran compounds such as 6′-[4-(4-anilinoanilino)ani- lino]-2′-chloro-3′-methylfluoran (13)7 giving images readable by near-infrared rays have also been developed for POS (points of sales) labels that are recently being watched with keen interest.
|
(13) |
This chapter describes the properties and syntheses of fluoran compounds, and their applications as well.
 23 августа, 2015
23 августа, 2015  Malyar
Malyar 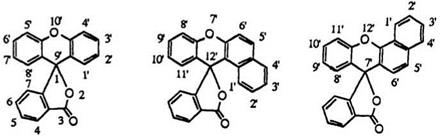
 Опубликовано в рубрике
Опубликовано в рубрике 