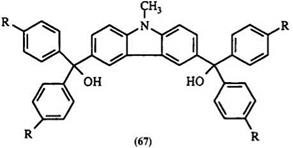
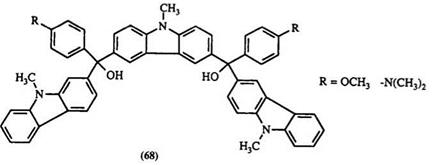
Formation of leuco compounds from ketones is generally a two-step process. The reaction of aromatic ketone with an arene nucleophile is normally carried out in the presence of excess POCl3 with P2O5, ZnCl2, or SOCl2. POCl3 serves as solvent and also as water acceptor. Usually dyes are formed directly in this reaction. Without isolation, they are treated with a base such as NaOH,77 NaOMe,92,M NaOEt,94 or a heterocycle such as imidazole78 to give the carbinol base, or the nitrogen substituent analogue, respectively. Benzophenone and substituted benzophenones containing an alkoxy or amino substituent are often used. Anilines, substituted anilines, and heterocycles such as N-ethylcarbazole,92 indole, 1,2-dimethylindole,40 tetrahydroquinolines, and quinoxaline93,95 have been used as nucleophiles. This reaction proceeds via intermediate 63 (Scheme 8). Replacement of the chlorine atom by the aromatic nucleophile gives the dye cation 64 followed by quenching with a base to give leuco compounds. For example, N — methyldiphenylamine reacts with N-methylcarbazole in this way to give the bis-triarylmethane leuco compound68 (e. g., 65a) and other similar compounds96-98 66-68.
|
|
|
|
|
|
Experimental Procedure96 for 4,4′-diethoxy-4"-[N-methyl-N-(4-cyano- phenyl)amino]-ethoxytriphenylmethane (65b). 14.1 g of P2O5 added at room temperature to a mixture of 4,4′-diethoxybenzophenone (13.5 g, 0.05 mol) and 4-cyanodiphenylamine (10.4 g, 0.05 mol) in 38.2 g of POCl3. The mixture was stirred at 40°C for 20h, poured into 500ml of ice water, and stirred at room temperature for about 10-15h until the dyestuff separated out as crystals. The crystals were isolated by filtration and, washed with water and dried in vacuum at 40°C to give 24.1 g (97%) dark red-violet crystals mp 69-75°C. 19.9g (0.04mol) of this dye in 200ml of EtOH was slowly added dropwise to 120ml of 1 M NaOEt solution at room temperature. The mixture was stirred at room temperature for 20 h, and filtered. The solvent was removed and the oily residue was stirred with 500ml of water and then cooled to 10 -15°C. The reaction mixture was filtered and the residue was dried in vacuum at room temperature to give 18.5g (91%) of colorless crystal mp 48-55°C.
 17 августа, 2015
17 августа, 2015  Malyar
Malyar 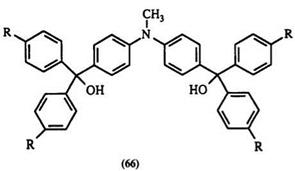
 Опубликовано в рубрике
Опубликовано в рубрике 