Diphenylmethanes that have two identical phenyl groups are synthesized by the condensation of formaldehyde or its equivalent with an arylamine in the presence of concentrated hydrochloric acid.47-51 However, it is usually difficult to stop the reaction at the diphenylmethane stage.
Magnesium phenolates react with triethylorthoformate regiospecifically at the ortho position of the phenoxy group (normally phenols give alkyl ethers) giving diarylmethanes. This reaction is complex and the product composition depends on the phenol and the reaction conditions.52
Reaction of 4-hydroxymethylaniline 37 with a variety of arylamines in the presence of an acid catalyst gives both symmetrical and unsymmetrical diphenylmethanes.53-55 This reaction proceeds via intermediate 38.
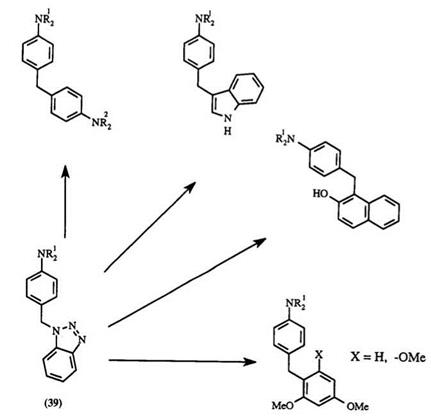
Aniline or N, N-dialkylanilines are readily alkylated by 1-(hyd- roxymethyl)benzotriazole to give 4-(benzotriazol-1-yl-methyl)anilines 39 (Scheme 6). Subsequent displacement of the benzotriazole group with
|
|
|
NR21= NH2 , NHMe, NMe2, NEt2 Scheme 6 |
arylamines or N, N-dialkylamines gives either symmetrical or unsymmetrical 4,4-methylene-bis-N, N-dialkylphenylamines.56,57
A general experimental procedure57 for a diarylmethane leuco compound via a benzotriazole: To a stirred solution of the corresponding (benzotriazol-1-yl-methy1)aniline (5 mmol) in methanol (30 ml) under reflux was added a solution of the appropriate aromatic compound (5 mmol) and concentrated hydrochloric acid (1 ml) in water (30 ml). The resulting mixture was heated under reflux followed by the addition of aqueous KOH (1 M, 50ml). The product was isolated by filtration or by extraction with ether, and further purified by recrystallization or by column chromatography.
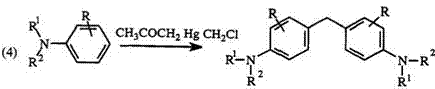
Oxidation of dimethylaniline with organometallic compounds such as osmiumcarbonyl,58 or palladium(II) compounds59 or f-butylperesters60 give bis(4-N, N-dimethylaminophenyl)methanes. The reaction of monohalogeno — alkyl mercurials with aromatic amines 40 in a molar ratio of 1:4 gives bis(4-aminophenyl)alkanes61 41 (Eq. 4).
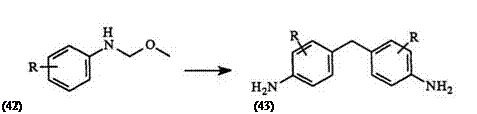
Treatment of N, 0-acetals 42 in which the para position is unsubstituted, with a twofold excess of the corresponding amine HCl in boiling 50% aqueous methanol gives rise to the bis(4-aminoary1)methane deriva — tive.62 43. This method is suitable only for 4-N, N’-unsubstituted diarylamines (Eq. 5).
(5)
R = 2-Me, H; 3-Me, 3-OMe
Reduction of benzophenones with FeC12/NaB(Et)3H63 or LiA1H464 or with NaBH4/CH3SO3H65 gives bis-phenylmethanes in low yields. Alternatively, bidentate or monodentate aromatic mercury salts reduce thioketones to diarylmethanes.66
Leuco diarylmethane compounds which have received a good deal of attention are the leuco auramines. Reaction of Michler’s hydrol with a
suitable amine, hydrazine, or sulfonamide gives auramine derivatives. These derivatives have low volatility with increased solubility in oil and show good stability in the leuco state.16
 12 августа, 2015
12 августа, 2015  Malyar
Malyar 
 Опубликовано в рубрике
Опубликовано в рубрике 