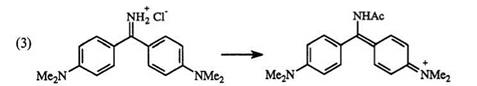
 |
Like most dyes, substituents on di — and triphenylmethane dyes have a significant effect on the absorption spectra. Acetylation of the imino nitrogen of auramine O (32) results in shift to longer wavelength (Eq. 3). A
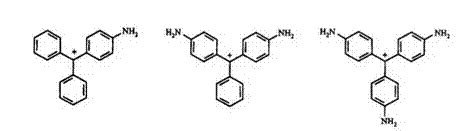
general rule for triphenylmethane dyes is that the greater the fraction of positive charge that is on the auxochromes, the longer the wavelength of absorption. A bathochromic shift can be achieved by introduction of electron-donating groups. For example, 4-aminotriphenylmethane 34 is orange yellow, 4,4′-diaminotriphenylmethane 35 is red-violet, and 4,4′,4"- triaminotriphenylmethane 36 is bluish red. Alkyl groups on nitrogen result in further bathochromic shift. Phenylation of amine nitrogen leads to even
greater bathochromic shifts. Replacement of the aromatic amino group with phenolic substituents results in hypochromic shifts. However, the sodium salt of phenolic dyes have similar absorption to the amine-substituted derivatives.
Steric effects play an important role in the absorption of triphenyl — methane dyes. Introduction of ortho substituents on the phenyl rings results in bathochromic shifts. This is due to the twisting of the phenyl rings resulting in a greater localized positive charge on the auxochromic nitro- gens.46 For example, tris(4-diethylaminopheny1)methane gives a purple dye on oxidation, while tris(4-diethylamino-2-methylphenyl)methane gives a blue material.
 12 августа, 2015
12 августа, 2015  Malyar
Malyar  Опубликовано в рубрике
Опубликовано в рубрике 