Some dyes can be formed from triphenylmethane leuco materials by simple thermolysis. For example, when 28 is heated an irreversible intramolecular alkylation reaction occurs to form the stable dye41 29 (Eq. 1).
|
|
|
Scheme 5 |
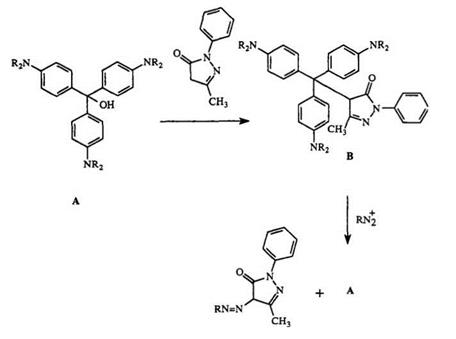
Besides the formation of colored compounds, leuco dyes can undergo other chemical reactions. For example, leuco bases can be sulfonated42 or nitrated.43 Leuco dyes containing phenolic groups can be converted into phosphonate esters by treatment with (PhO)3P or (PhO)2P—OH.44 Triphenylmethanecarbinols react with phenylmethylpyrazoles to form C—C bond compounds of type B (Scheme 5). The carbinol can be regenerated on treatment with a diazonium salt. Furthermore, the addition of Grignard
|
|
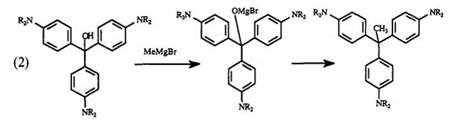
reagents to triphenylmethane carbinols forms 31A via a Tserevetinov reaction (Eq. 2). Triphenylmethane carbinol methyl ethers of 31 undergo similar reactions with Grignard reagents.45
 12 августа, 2015
12 августа, 2015  Malyar
Malyar 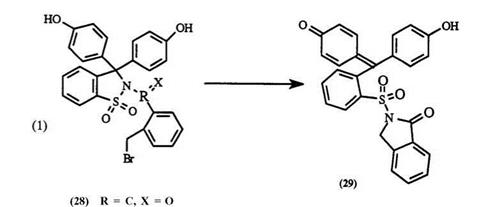
 Опубликовано в рубрике
Опубликовано в рубрике 