The formation of color from triheteroarylmethanes differs from the methodology employed for triphenylmethane leuco dyes40 (Scheme 4). Dyes are initially formed by alkylation of the pyridyl nitrogen, followed by deprotonation at the central methine carbon. Thus, treatment of the colorless 3,3′-diindolyl-4-pyridylmethane 22 with excess methyl iodide produces colorless compound 23. Subsequent treatment of 23 with hydroxide
|
|
yields a yellow dye 24, which undergoes rapid oxidation to give a blue dye 26 followed by slow dealkylation to yield the red dye 27.
 11 августа, 2015
11 августа, 2015  Malyar
Malyar 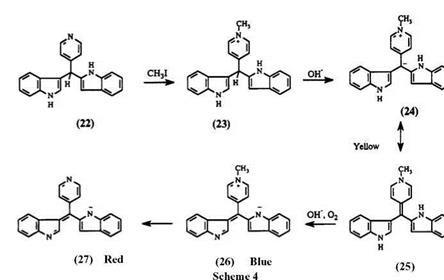
 Опубликовано в рубрике
Опубликовано в рубрике 