In analogy to the original preparation of Malachite Green lactone, pyromellitic anhydride has also been reacted with N, N-dimethylaniline in a zinc chloride melt to yield mixtures of the bisphthalides 29 and 30.109 However, far superior yields were obtained if reaction was carried out in two steps, as described in Scheme 12. The initial condensation was carried out
|
|
|
|
in the presence of aluminum chloride and the second in acetic anhydride leading to an approximately 1 : 1 mixture of isomers 29 and 30. By utilizing different dialkylanilines in the second condensation, asymmetrically substituted compounds were also prepared. These color formers produce greenish-blue images on application to clay developers.
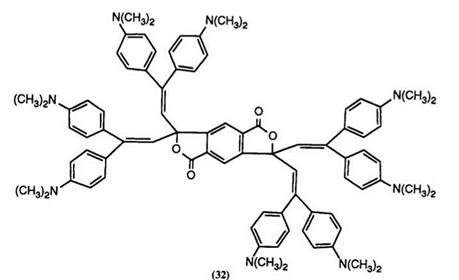
Reaction of pyromellitic anhydride with 1,1-bisdimethylaminophenyl- ethylene has been shown110 to yield a mixture of the bisphthalides 31 and 32 which are infrared-absorbing color formers on clay.
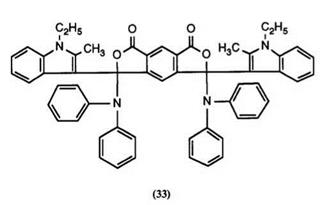
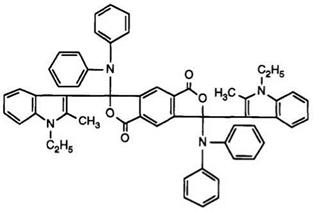
Finally, 1-ethyl-2-methyl indole was reacted111 with pyromellitic anhydride to give a mixture of keto acids, as in Scheme 12, which was then treated with diphenylamines to yield bisphthalides such as 33 and 34. These color formers produce orange images on development, as also do those in which the indole residue is replaced by a 4-dialkylaminophenyl group.
|
|
|
|
(34)
 8 августа, 2015
8 августа, 2015  Malyar
Malyar 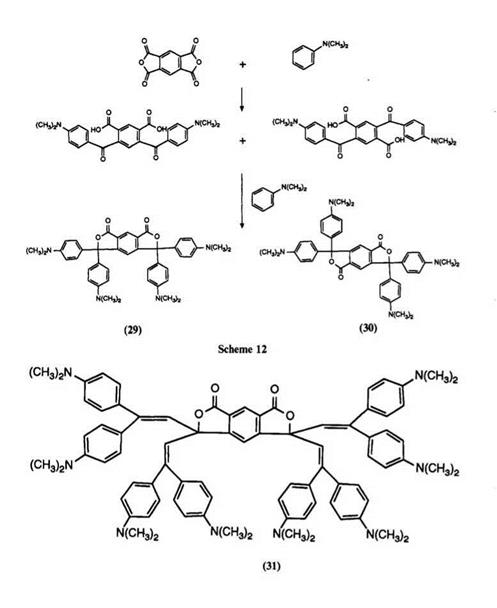
 Опубликовано в рубрике
Опубликовано в рубрике 