One further example of this principle of extended conjugation to produce infrared-absorbing color formers is the introduction of the bu — tadienyl group at the 3-position of the phthalide ring.100 Thus, 2-(4- dimethylaminobenzoyl)benzoic acid was reacted with 1,1-bis(4-dimethyl — aminophenyl)buta-1,3-diene in acetic anhydride to yield the phthalide 24. This idea, however, does not appear to have been greatly exploited, probably due to the fact that the ring-closed phthalides themselves are colored. The only other report101 to date describes analogues of 24 in which
|
(24) |
the 3-dimethylaminophenyl group is replaced by heterocycles such as carbazole, indole, tetrahydroquinoline, or julolidine.
 5 августа, 2015
5 августа, 2015  Malyar
Malyar 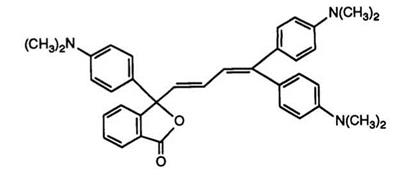
 Опубликовано в рубрике
Опубликовано в рубрике 