As in the previous section, one class of compounds, namely, the 3-dialkylaminophenyl-3-indolyl-4-azaphthalides, is of primary importance from a commercial viewpoint. The 7-azaisomer, the first dialkylamino- phenylindolylazaphthalide, has been prepared69 as shown in Scheme 8 (cf. Scheme 7).
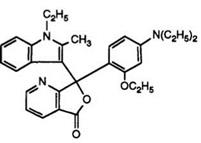
Heating pyridine-2,3-dicarboxylic acid anhydride with 1-ethyl-2- methylindole has been claimed to yield solely the pyridine-2-carboxylic acid, albeit in low yield. This then clearly reacts with N, N-diethyl-3-toluidine in acetic anhydride to give the 7-azaphthalide. This is surprising in view of a later report70 in which a one-pot process has been described. Heating pyridine-2,3-dicarboxylic anhydride, prepared in situ, with the indole and subsequent reaction with 3-N, N-diethylamino-phenetol under identical conditions to those used in Scheme 8 (but without intermediate isolations) produced a 20:1 mixture of the 4- and 7-azaisomers 16 and 17. It appears that in the previous report the major intermediate isomer, the pyridine-3- carboxylic acid, has not been isolated.
Color formers such as 16 and 17 and their mixtures are commonly known as “Pyridyl Blues” and are excellent products in combination with organic developers, yielding intense blue images with very high fastness properties. However, it has been observed71 that, for some applications, the 7-azaisomer is too reactive and hence it was desirable to modify the
|
Scheme 8 |
|
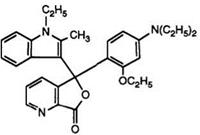
(17)
synthesis to produce solely the 4-azacompound 16. It was later discovered72 that the addition of catalytic quantities of metal salts such as zinc or aluminum chlorides during condensation of pyridine-2,3-dicarboxylic acid anhydride with indole leads solely to formation of the pyridine-3-carboxylic acid, consequently achieving the desired result of eliminating formation of the 7-azaisomer. It is interesting to note that, when the 2-position of the indole ring is unsubstituted, the use of a large excess of aluminum chloride in the initial condensation is reported73 to lead to exclusive formation of the 7-azaisomer.
Due to the commercial importance of the Pyridyl Blues, it is not surprising that a considerable number of structural variants have been patented. Variations in the aniline part of the molecule have included diarylaniline,74 long-chain alkoxy-substituted anilines,75 cyclohexyl-
anilines,76 and amino-substituted anilines,77 while long-chain alkyl groups have also been attached to the indole nitrogen atom.78 Compounds carrying substituents in the pyridine ring have also been reported.79
Replacement of the pyridine-2,3-dicarboxylic acid anhydride by the corresponding 3,4-dicarboxylic acid anhydride leads to formation of approximately 1 : 1 mixtures of the 5- and 6-azaisomers.80 These blue color formers are suitable for use with all types of developers. A number of heterocycles other than indoles have also been used to provide novel color formers. Thus, carbazoles, acridines, and tetrahydroquinolines have all been shown81 to yield the corresponding 4- and 7-azaphthalides as color formers. Indolizines give rise to green color formers,65 while bridgehead nitrogen heterocycles such as imidazo[1,2-a]pyridines produce 4-azaphthalides66 forming blue images.
Pyrazine-2,3-dicarboxylic acid anhydride has also been shown69 to react according to Scheme 8, yielding 4,7-diazaphthalides very similar in
their properties to the Pyridyl Blues, as is the case with quinoxaline-2,3- dicarboxylic acide anhydride,82 which also has been reacted66 with imidazo — [ 1,2-ajpyridines to produce green color formers.
 2 августа, 2015
2 августа, 2015  Malyar
Malyar 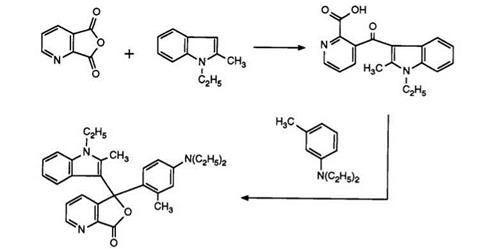
 Опубликовано в рубрике
Опубликовано в рубрике 