4.2.1. Triarylmethane Phthalides
Since the introduction of carbonless copying papers, CVL has undoubtedly found the most widespread use as a phthalide color former. Hence, it is appropriate to describe in detail the various approaches to its synthesis, particularly as they are representative of the preparations of various other phthalides described herein.
 (CH3)2n
(CH3)2n
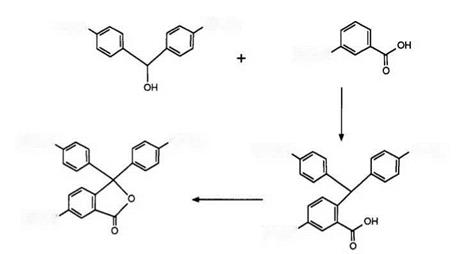
The first synthesis of CVL, as described by NCR,2 is shown in Scheme 2. The condensation of 4,4-bisdimethylaminobenzhydrol with 3-dime — thylaminobenzoic acid was accomplished in 90% sulfuric acid to yield 2-(4,4-bisdimethylaminobenzhydryl)-5-dimethylaminobenzoic acid (leuco
CVL), which was subsequently oxidized to CVL using lead dioxide in dilute mineral acid. Since this time, numerous variations on both reaction conditions and also reagents employed for this synthesis have been described. For example, it has been shown5 that both yield and quality of the intermediate leuco CVL may be improved by carrying out the initial condensation in dilute mineral acid, whereby the particle size of the benzhydrol has also been claimed6 to be of importance. The oxidation step has been carried out using potassium permanganate,7 potassium persulfate,8 potassium ferricyanide,9 or chloranil in the presence of a metal complex catalyst.10 However, the method of choice would appear to be the use of hydrogen peroxide in basic solution,11 — 14 catalyzed by copper or cobalt complexes,15 chromium, iron, manganese, or vanadium salts16 and iron or manganese chelates17 having been claimed to be advantageous. Furthermore, the oxidation has been effected using air or oxygen in the presence of cobalt or manganese complexes,18 acetic acid,19 or iron, copper, or cobalt salts in acidic media.20 One further variation on the above synthesis has been the replacement of 4,4′-dimethylaminobenzhydrol by other substrates. The corresponding amino derivative, leucauramine, has also been found21 to react analogously, while the direct formation of leuco CVL by condensation of N, N-dime — thylaniline, 4-dimethylaminobenzaldehyde, 3-dimethylaminobenzoic acid, and urea in strong acid has been reported.22 Finally, urea has been reacted
N(CHj)2
(CH3)2N
(CHjJjN
Scheme 3
with 4-dimethylaminobenzaldehyde and N, N-dimethylaniline to yield a mixture of mono — and bis(4,4′-dimethylaminodiphenylmethyl) ureas which also yield leuco CVL on reaction with 3-dimethylaminobenzoic acid.23
The most versatile route to lactone color formers is based on the elegant synthesis of Malachite Green lactone described by Haller and Guyot in 18 99.24 Scheme 3 illustrates the application of this route for the preparation of CVL.
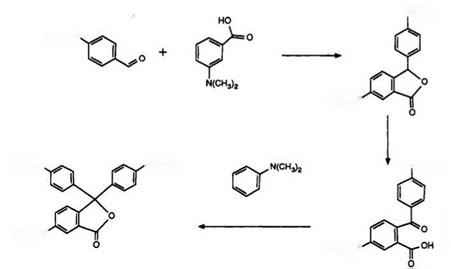 4-Dimethylaminobenzaldehyde condenses with 3-dimethylaminoben — zoic acid in the presence of acetic anhydride to yield 3-(4-dimethylamino — phenyl)-6-dimethylamin0phthalide.25 Subsequent oxidation, preferably with 3-nitrobenzenesulfonic acid,26 yields the bisdimethylaminobenzophenone — carboxylic acid, which then is treated with dimethylaniline in acetic anhydride to form CVL.26’27 A variation for the preparation of the intermediate phthalide by treatment of 2-formyl-5-dimethylaminobenzoic acid with dimethylaniline has also been described.28 However, the difficulty in preparing this former starting material renders this route less attractive. It has also been shown29 that treatment of the phthalide intermediate with dimethylaniline in the presence of a Friedel-Crafts catalyst yields leuco CVL, which may then be oxidized as described for Scheme 2. The development of CVL as a color former for carbonless copying papers was a result of the deficiencies of the readily available Malachite Green lactone, orig-
4-Dimethylaminobenzaldehyde condenses with 3-dimethylaminoben — zoic acid in the presence of acetic anhydride to yield 3-(4-dimethylamino — phenyl)-6-dimethylamin0phthalide.25 Subsequent oxidation, preferably with 3-nitrobenzenesulfonic acid,26 yields the bisdimethylaminobenzophenone — carboxylic acid, which then is treated with dimethylaniline in acetic anhydride to form CVL.26’27 A variation for the preparation of the intermediate phthalide by treatment of 2-formyl-5-dimethylaminobenzoic acid with dimethylaniline has also been described.28 However, the difficulty in preparing this former starting material renders this route less attractive. It has also been shown29 that treatment of the phthalide intermediate with dimethylaniline in the presence of a Friedel-Crafts catalyst yields leuco CVL, which may then be oxidized as described for Scheme 2. The development of CVL as a color former for carbonless copying papers was a result of the deficiencies of the readily available Malachite Green lactone, orig-
inally prepared1 by condensation of phthalic anhydride with dimethylaniline in the presence of zinc chloride, as a color former. The green color was unsuitable30 and the intensity of the image insufficient with certain developers. The introduction of the dimethylamino group into the 6-position results in a blue image with increased intensity.
However, despite the commercial importance of CVL as a color former, the product possesses a number of disadvantages, notably poor lightfastness especially in combination with clay developers, and low solubility in organic solvents. Hence, it is not surprising that a vast number of variations on the basic structure have been described in the patent literature, typical examples of which are structures 3-5 (see also for example Ref. 26).
|
|
Compounds 3 and 4 are claimed31,32 to exhibit good solubility, while 5 is stated33 to possess excellent light-resistance, However, to date, no similar product has been able to replace CVL in the marketplace. One further example of the flexibility of the synthetic route in Scheme 3 is the preparation of compound 6,34 which is reported to show light absorption in the near infrared region and is thus suitable for recordings readable by lasers.
|
|
|
|
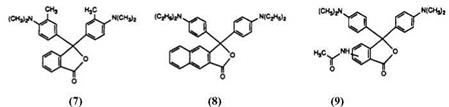
The introduction of the dimethylamino group into the phthalide ring has, however, not been the sole attempt to modify the properties of Malachite Green lactone. Early attempts resulted in the preparation of compounds such as 7-9. Compounds 7 and 8 were prepared by condensation of the appropriate amine with phthalic anhydride35 and 2,3-naphthoic anhydride,36 respectively, with the appropriate aniline in the presence of zinc chloride. Compound 9 was prepared by nitration of Malachite Green lactone, yielding a mixture of isomers which were subsequently reduced and acetylated.37 None of these modifications, however, gave rise to the required blueshift. It is perhaps noteworthy that reductive alkylation of a mixture of nitromalachite green lactones with palladium on charcoal and formaldehyde has been proposed38 as a synthetic route to CVL. This process, however, is clearly of little synthetic value since it not only yields mixtures, but also reduces the lactones to the leuco compounds which subsequently must be reoxidized.
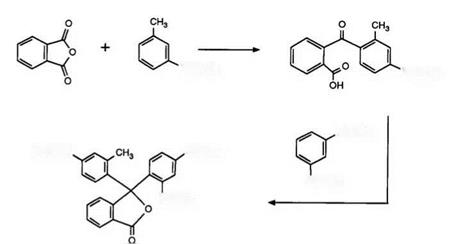
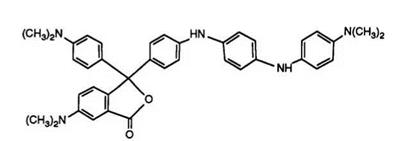
It thus became clear that the improvement in properties of CVL as opposed to Malachite Green lactone was due to the electron-donating effect of the dimethylamino group in the 6-position of the phthalide ring. On this basis a far superior approach to that above was the incorporation of additional electron donors into the dialkylaminophenyl groups.39 Scheme 4 demonstrates the synthetic route to such compounds.
Phthalic anhydride condenses with the aniline derivative in the presence of zinc or aluminum chlorides to yield the intermediate benzoyl- benzoic acid, which subsequently reacts with 1,3-bis-N, N-dimethylaniline in acetic anhydride to yield the phthalide. The above compound gives a violet-gray image when applied to a clay developer. Clearly this synthesis is also very flexible and variations in shades of color formers have been obtained by varying the aniline components and also by using phthalic anhydrides substituted, for example, by nitro groups or chlorine atoms. Such products have excellent properties as color formers and have been used commercially. Furthermore, this synthetic route is of great importance for the preparation of heterocyclic substituted phthalides, as will be seen later.
 Scheme 4
Scheme 4
 26 июля, 2015
26 июля, 2015  Malyar
Malyar 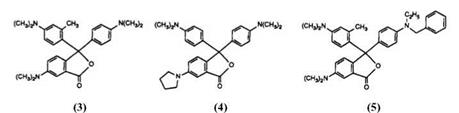
 Опубликовано в рубрике
Опубликовано в рубрике 