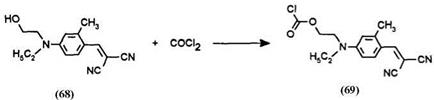
Leuco Methylene Blue, Basic Blue 3, or phenazine dyes are capped with a dye bearing acid chloride or chlorocarbonyl functionality. Normal procedures employed for the synthesis of benzoyl leuco Methylene Blue can be utilized except that a dye chloroformate (69) replaces the benzoyl chloride.
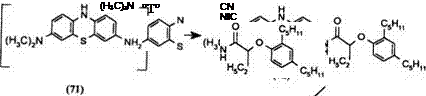 |
The exocyclic amino group of Azures A or B can be used to attach a long aliphatic ballasting group. Under mild reaction conditions, the ring amino group at the 10-position being much less reactive remains unsubstituted. The dye capping is effected at reflux temperatures in an organic solvent.
Azure A can be reduced to an air sensitive intermediate 71 and acylated in one step to produce the ballasted leuco 72 which can be isolated and capped with the dye chloroformate 69 to give the ballasted yellow dye release developer 73.
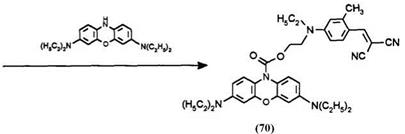
Derivatives of Methylene Violet 6 possessing long aliphatic chains are obtained by oxidative coupling of 3-acetoxyphenothiazine with a secondary amine in the presence of an oxidant such as iodine. The oxidative coupling of phenothiazine with amine is well known but in this case the reaction does not stop there but proceeds further at reflux temperatures to the phenothiazinone 74.9 Reduction of the latter dye and treatment with acetic anhydride yields the ballasted phenothiazine 6. Reaction of 75 with the dye chloroformate 70 yields the ballasted leuco dye developer 76.
|
|
Synthetic Method 13: 4-{ethyl-[2-(3,7-bisdiethylaminophenoxazinyl)- carbonyloxyethyl]amino}-2-methylphenylmethylenepropanedinitrile(70) (procedure from US. Patent 4,981,775).22 The yellow dye {[4-ethyl-(2-hydro- xyethylamino)]-2-methylphenyl}methylenepropanedinitrile (68) (2.55 g, 0.01 mol) was dissolved in 70ml of dichloromethane and phosgene 12.5% w/w solution in toluene (16 g, 0.02 mol) was added. After stirring for 2 h at room temperature, the solvent was evaporated and the residue recrystallized from a mixture of dichloromethane-ether to give 3 g of the dye chloroformate 69 as yellow leaflets.
Basic Blue 3 (Aldrich Chemical Co., 85% pure: 12.7 g, 0.03 mol) was dissolved in 200ml of water and 200ml of dichloromethane was added to form a two-phase mixture. The mixture was stirred gently under nitrogen gas and the pH was adjusted to 10 with a 40% aqueous NaOH solution. Sodium dithionite (6.75 g, 0.033 mol) in 100ml of water was added and the mixture stirred for 10 min as decoloration took place. The pH was readjusted to 6 and a solution 7.7 g (0.03 mol) of the dye chloroformate 69 in 100ml of dichloromethane was then added in one portion. The mixture was stirred for 2;Т h, the pH being continuously adjusted to 6 with 40% aqueous NaOH solution. The pH was raised to 10 and the mixture was filtered through a shallow plug of Hyflo Supercel filter aid. The organic layer was separated, washed with brine, and dried over magnesium sulfate. Silica gel 60 (10 g) was added to the dried solution and the filtered solution was then concentrated to dryness to yield a yellow-brown foamy solid (15.9 g). The solid was triturated with 250ml of boiling isopropanol and dried to yield 14.24 g of the dye-capped leuco 70.
 25 июля, 2015
25 июля, 2015  Malyar
Malyar 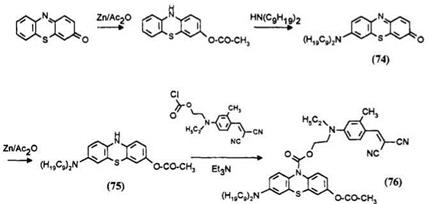
 Опубликовано в рубрике
Опубликовано в рубрике 