 |
Phenazine leuco dyes, like their thiazine and oxazine analogues, have also found application in electrolytic recording, overhead transparencies, and Color Dry Silver. The phenazine leuco 48 is employed in electrolytic recording,17 whereas compounds 49, 50 are described as useful in thermographic and photothermographic systems. 18
Phenazine leucos are generally more reactive and more susceptible to air oxidation than the thiazines and oxazines. Incorporation of electron — withdrawing groups on the acyl substituent at the 10-position of the leuco dye can provide a substantial improvement in the thermal and light stability of the leuco form and it is found that in general the stronger the electron — withdrawing character of the acyl substituents the more stable the leuco is.18
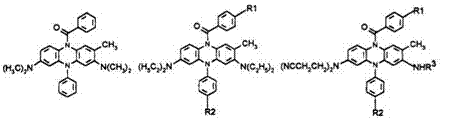
Phenazine leucos are capable of providing yellow, orange, red, and magenta images whereas thiazine and oxazine leucos are normally restricted to turquoise, blue, and purple colors.19 Color depends on the electronic nature of substituents R1 to R4, as shown in Table 3.
Table 3. Effect of Substituents on the Color of Phenazine Dyes
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
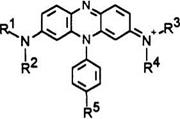
Phenazine leucos until now are usually substituted at their 3 and 6 positions by amino groups due to the normal method of synthesis of the parent phenazine dyes. These types of leuco dyes are reactive. An alternative method of dye synthesis allows access to phenazine dyes with just one substituent at the 3-position.20 The resulting leuco dyes are called half diazine leucos. The loss of one exocyclic amino group leads to higher redox potential and results in less reactive leuco dyes, more useful in applications such as thermographic and photothermographic imaging, particularly Color Dry Silver.
The new phenazine dyes are prepared according to established procedures with some modifications:
|
|
Synthetic Method 8: 9,10-dihydro-9-phenyl-10-(4-phenylsulfonyl-
benzoyl) -3,6-di(N, N-diethylamino)phenazine (procedure from U. S. Patent 4,889,932) 1 Into a 1-liter, three-necked round-bottom flask equipped with a mechanical stirrer, pH electrode, and an argon inlet and outlet, was added 10 g (0.023 mol) of tetraethylphenosafranine and 200 ml of deionized water. The mixture was stirred for several minutes to dissolve the dye as completely as possible. Methylene chloride (40 ml) was then added, and the system was flushed with argon. The pH was adjusted to 10 with 20% aqueous NaOH, and 8 g (0.046 mol) of sodium dithionite was added all at once as a solid. The pH dropped to between 3 and 4 and the solution turned from purple to olive-green over several minutes. The solution was stirred for about 20 min. The acid chloride, 9.69 g (0.035 mol) of p-phenylsulfonylben- zoylchloride, ground to a fine powder and suspended in 60 ml of methylene chloride, was added dropwise over a period of 30-45 min., the pH being adjusted continuously with NaOH solution to keep it between 3 and 4.5.
After the addition of acid chloride, about 25 ml of methylene chloride was used to rinse the residual acid chloride into the flask. The mixture was stirred for about 3 h. The pH was then adjusted to between 9.5 and 10 and the solution was stirred for an additional hour. The organic layer was separated from the aqueous layer and washed once with 5% NaOH solution. The solution was dried over calcium sulfate and the methylene chloride was evaporated leaving about 10.36 g (69%) of crude product. Decolorizing three times with Attapulgus clay yielded a light yellow brown methylene chloride solution which, on removal of solvent, gave about 5.2 g of product (35%).
Synthetic Method 9: N-[8-bis(2-cyanoethyI) -9-[4- (phenylsulfonyl) — benzoyl]-9, 10-dihydro-1 0-phenyl-2-phenazinyl]-4-phenylsulfonylbenzamide (53) (procedure from US. Patent 4,889,932).18 A 3-liter round-bottom flask fitted with a mechanical stirrer was loaded with 16.6 g (0.066 mol) of 4-[di(2-cyanoethyl)amino]aniline in 800 ml of deionized water. A 10% excess of aniline (13.56 g, 0.1456 mol) and 100ml of deionized water were added to the mixture. The mixture was cooled to 0°C in an ice bath, and 10 ml of concentrated hydrochloric acid in 25 ml of water was added. Then 6.31 g (0.083 mol) of sodium dichromate in 25 ml of water was added. The temperature rose to 7°C. Stirring was continued as 9ml of concentrated hydrochloric acid in 25 ml of distilled water was added over a period of 2 h. After 16 h the temperature had risen to 20°C. The mixture was heated under reflux for 4 h and then filtered hot. The filter cake was washed with 1.5 liters of boiling water. The combined filtrates were concentrated to 1.4liters by vacuum evaporation, and then heated to 75°C as 200g of sodium chloride was added. The mixture was cooled to room temperature, chilled in an ice bath, and the solid was then recovered by filtration to give 14.6g (yield = 51.6%), X max = 537nm in methanol.
A 1-liter three-necked flask was fitted with a Claisen head equipped with two dropping funnels, a mechanical stirrer, and a pH electrode. A solution containing 5g (0.012 mol) of the dye prepared above, 210 ml of water, and 0.2 g of ethylenediaminetetraacetic acid was added to the flask and stirred, while 250 ml of methylene chloride was added. The system was closed and flushed with argon, the pH was adjusted to 10, then 2.44 g (0.014 mol) of sodium dithionite was added. The solution turned orange and the pH dropped to 3.7. Aqueous 25% NaOH solution was added to bring the pH to 4.5, and then 7.53 g (0.027 mol) of 4-phenylsulfonylbenzoylchloride in 60 ml of methylene chloride was added dropwise. After 1^ h the pH was raised to 10-11. The methylene chloride layer was separated and dried over
calcium sulfate. The solution was treated with about 10 g of Attapulgus clay and filtered. The solvent was then evaporated, leaving 8.14 g of solid (79%).
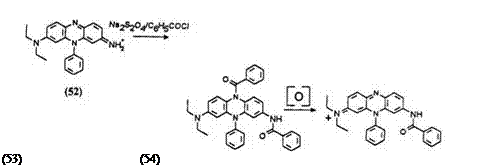
As in the case of thiazine and oxazine leuco dyes described earlier, the reductive acylation of the phenazine dye 52 results in the acylation of the exocyclic amino group.18 The phenazine leuco obtained 53 retains the exocyclic amide group on oxidation resulting in the acylated phenazine dye 54, the color of which is different from the one intended.
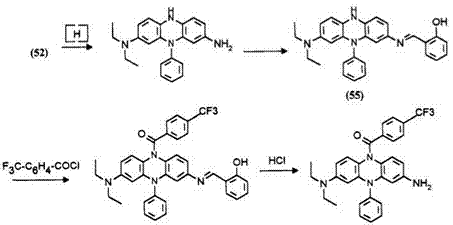
An alternative method has been described in which the exocyclic free amino group of the leuco dye is protected with salicylaldehyde.20 The resulting leuco 55 is acylated at its 10-position 56 and the salicylalimine protecting group is hydrolyzed with hydrogen chloride leaving a phenazine leuco dye possessing an exocyclic free amino group 57. This leuco dye regenerates the original phenazine dye 52 on oxidation.
Synthetic Method 10: 3-amino-4-diethylamino-9-phenyl-10- (2-trifluoro- methylbenzoyl)-9,10-dihydrophenazine (57) (procedure from Japanese Patent 63-112569).20 3-Amino-6-diethylamino-9-phenylphenazinium chloride (Heliotrope BS) (18.9g) was dissolved in 350ml of hot water at 80°C. To the solution cooled to 70°C was added 350 ml of toluene, followed by 43.5 g of sodium dithionite and 20g of a 20% aqueous NaOH solution. The mixture was stirred vigorously and purged with a stream of nitrogen. After the color of the solution had completely discharged, 13 ml of glacial acetic acid was added to give a pH of 4 and 15.3 g of salicylaldehyde was added over 30 min at 60°C. The mixture was stirred at that temperature for 3 h and cooled down, then 9 g of NaOH was added, followed by 26.1 g of 2-trifluoromethyl-benzoylchloride over 1 h at 20-30°C. The mixture was stirred for 3 h and filtered. The filtrate was separated and the toluene layer was stirred with 250ml of 1 N hydrochloric acid for 30 min at 30-35°C to hydrolyze the imine. The aqueous layer was also stirred with 1 N HCl. The combined aqueous layers were washed with chloroform and neutralized with NaOH. The precipitate was extracted with 150ml of toluene and the organic layer was washed with water, dried, treated with activated charcoal, and concentrated to dryness. The residue was recrystallized to yield 14.9 g of 3-amino-6-diethylamino-9-phenyl-10-(2-trifluoromethylben- zoyl)-9,10-dihydrophenazine having a melting point of 139.5-143°C.
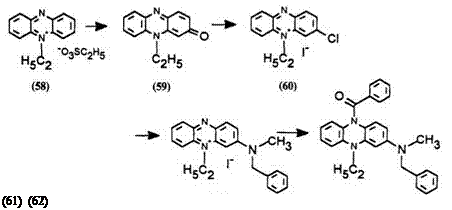 |
Synthetic method 11: 3-(N-benzyl-N-methyl)amino-9-ethyl-10-ben — zoyl-9,10-dihydrophenazine (62) (procedure from EP Patent Application 671,393).21 9-Ethylphenazinium ethosulfate (58) (obtained from phena-
zine and diethylsulfate) (62 g) was dissolved in 2.5 liters of water and 125 g of potassium ferricyanide was added, then 327 ml of a 10% aqueous NaOH was added slowly. The solution turned dark red. After standing overnight, 300ml of a 40% aqueous NaOH was added. The resulting precipitate was
collected by filtration, washed with water, and dried to give 36.42g (88%) of 9-ethyl-3-phenazinone (59).
Seventeen grams of phenazinone 59 was dissolved in 80ml of phos — phoryl chloride. Phosphorous pentachloride (15.6 g) was added and the mixture stirred for 2 h. The product (chloride salt) was collected by filtration, washed with ether, and dried. This solid was dissolved in 500ml of water, filtered to remove some insoluble tar, and 17.9g of potassium iodide was added to precipitate the iodide. The product was collected by filtration, washed with water, and dried in vacuo to yield 19.9g (71%) of 3-chloro-9-ethylphenazinium iodide (60).
The chlorophenazinium salt 60 (15.5 g) was dissolved in 2.5 liters of acetonitrile, filtered, and 12.6 g of benzylmethylamine was added. The mixture was stirred for 20h and solvent was removed by rotary evaporation. The resulting solid was dissolved in 600ml of ethanol and reprecipitated by pouring into 3 liters of ether to yield 11.1 g (58%) of the magenta dye 3-(N-benzyl-N-methyl)amino-9-ethylphenazinium iodide (61).
Twelve grams of the magenta dye 61 was dissolved in 250ml of methylene chloride and stirred gently with a solution of 12.2g of sodium dithionite in 250 ml of water. A solution of 5 g of benzoyl chloride in 10 ml of dichloromethane was added slowly to the lower organic layer. The pH of the upper aqueous layer was maintained at 5 to 6. The organic layer was separated, washed with dilute aqueous NaOH and brine. The solution was absorbed onto silica gel and rotary evaporated to dryness. The product was washed from the silica with ether. The ether solution was evaporated to yield 8.2 g of the leuco dye 3-(N-benzyl-N-methyl)amino-9-ethyl-10-ben — zoy1-9,10-dihydrophenazine (62).
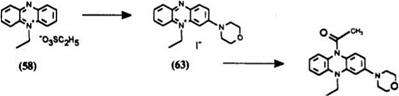
Synthetic Method 12: 3-morpholino-9-ethyl-10-acetyl-9,10-dihydrophe — nazine (65) (procedure from EP Patent Application 671,393).21 9-Ethyl — phenazinium ethosulfate (58) (7.69 g, 23 mmol) was weighed into a 1-liter three-necked round-bottom flask and dissolved completely in 400 ml of ethanol. Air, dried by passage through concentrated sulfuric acid, was bubbled through the solution. Morpholine (2.0 g, 23 mmol) was then run into the stirred solution. A magenta dye began to form immediately. After 2 h of air bubbling through the solution, solvent evaporation gave a tar which crystallized on standing. The ethosulfate salt was dissolved in 750 ml of water and the stirred solution treated with 250g of sodium iodide. Stirring was continued for 30 min. The precipitate was collected by filtration, washed with a little cold water, and then with a minimum volume of acetone, and dried in vacuo at 70°C to afford 6.51g (67%) of the dye 3-morpholino-9-ethylphenazinium iodide (63): ^max(EtOH) 526 nm (s 1.62 x 104).
|
|
The above dye (63) (0.1264g, 3 mmol) and 0.261 g (4 mmol) of zinc powder was weighed into a 100ml round-bottom flask, which was sealed with a rubber septum and purged with a stream of nitrogen. Anhydrous tetrahydrofuran (20 ml) was introduced and the suspension was degassed for a further 30 min. Glacial acetic acid (0223 g, 4 mmol) was introduced by syringe. On stirring vigorously, the color of the solution faded to a yellow solution. After 30 min, 0.44 g (4.3 mmol) of triethylamine was added, followed by 0.77 g (10 mmol) of acetyl chloride. The solution was left to stir overnight at room temperature under nitrogen. After 19 h, 1 g (10 mmol) of triethylamine was added by syringe. Water (10 ml) was then added slowly, followed by 20 ml of chloroform. The mixture was exposed to air only, once this point had been reached. The aqueous phase was separated, filtered, and washed once with 20 ml of chloroform. The combined organic extracts were washed with water (3 x 25ml) until no trace of dye remained, then dried over magnesium sulfate. Solvent evaporation gave an oil (1.117 g) which crystallized on standing. Recrystallization from ethylacetate-ether afforded 0.5 g (56%) of the leuco dye 3-morpholino-9-ethyl-10-acetyl-9,10-dihyd — rophenazine (64) as faintly colored platelets (mp 161-162°C).
 21 июля, 2015
21 июля, 2015  Malyar
Malyar 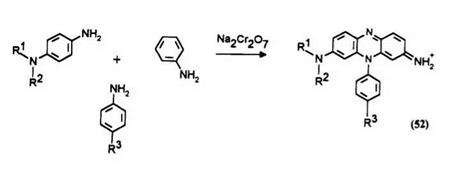
 Опубликовано в рубрике
Опубликовано в рубрике 